A two-dimensional cuco 2 the s 4 Nanosheets and their preparation methods and applications as electrocatalysts in oxygen reduction and oxygen evolution reactions
A nanosheet and reaction technology, applied in the field of nanomaterials and its preparation and application, to achieve the effects of excellent performance and stability, low overpotential, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0033] Example 1 Two-dimensional CuCo 2 S 4 Preparation of nanosheets
[0034] At room temperature, in a clean, dry 250mL three-necked flask, add 0.08g Cu(acac) 2 and 0.15g Co(acac) 2 Solid, 10mL DDA, ultrasonic dispersion, the above mixture at 5 ℃ min -1 The heating rate was heated to 120°C, and after 30min of incubation, 1.2mL of DDT was added, and continued at 5°C for min -1 The heating rate was raised to 245 °C, kept at this temperature for 10 minutes, then stopped, cooled naturally to room temperature, centrifuged, washed, and two-dimensional CuCo 2 S 4 Nanosheets.
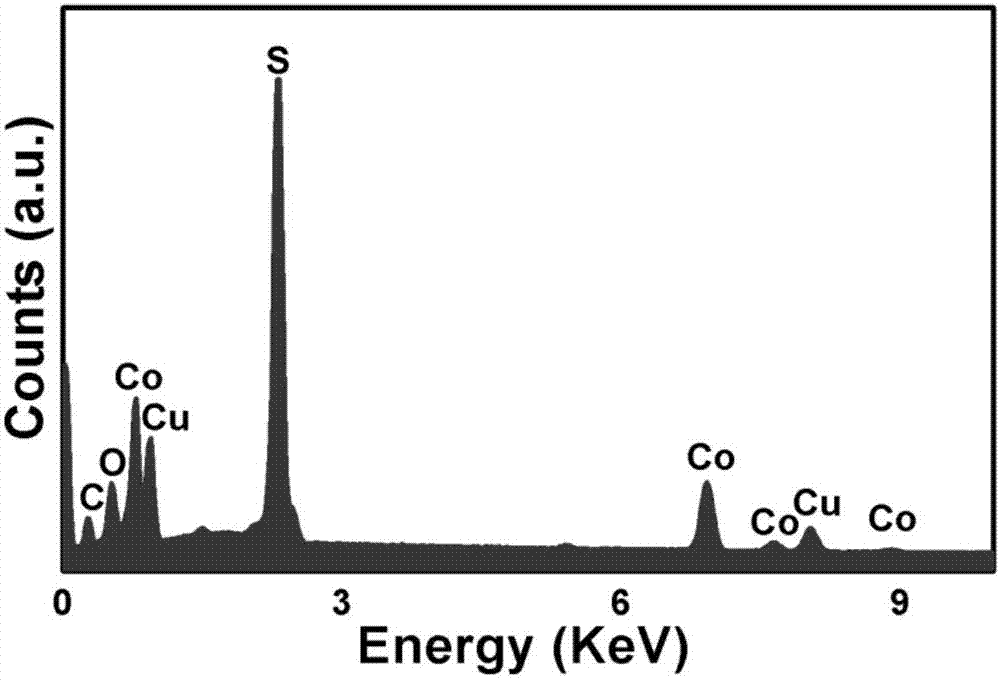
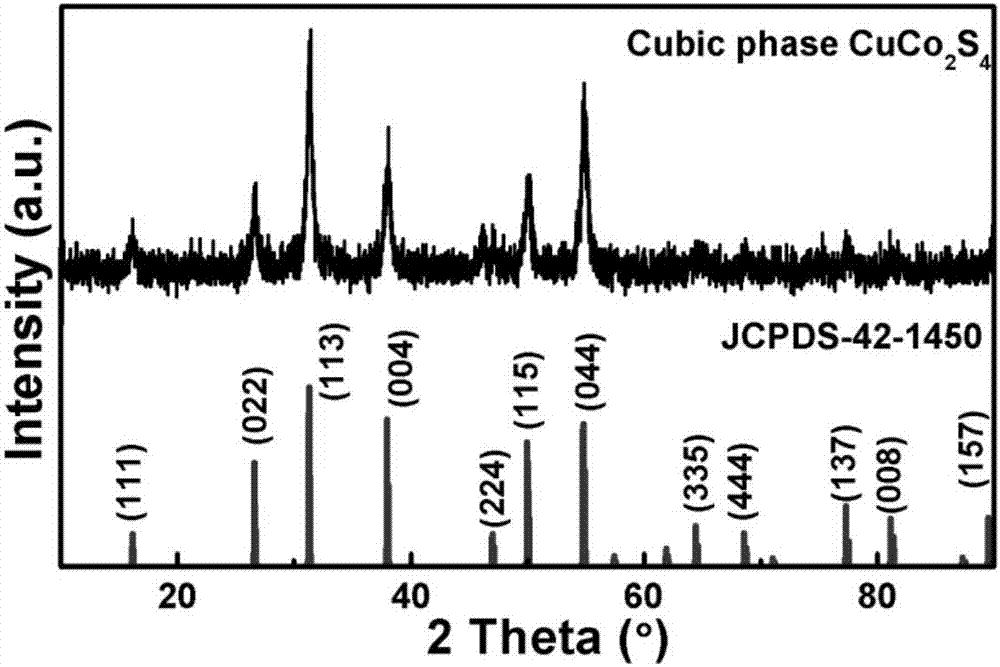
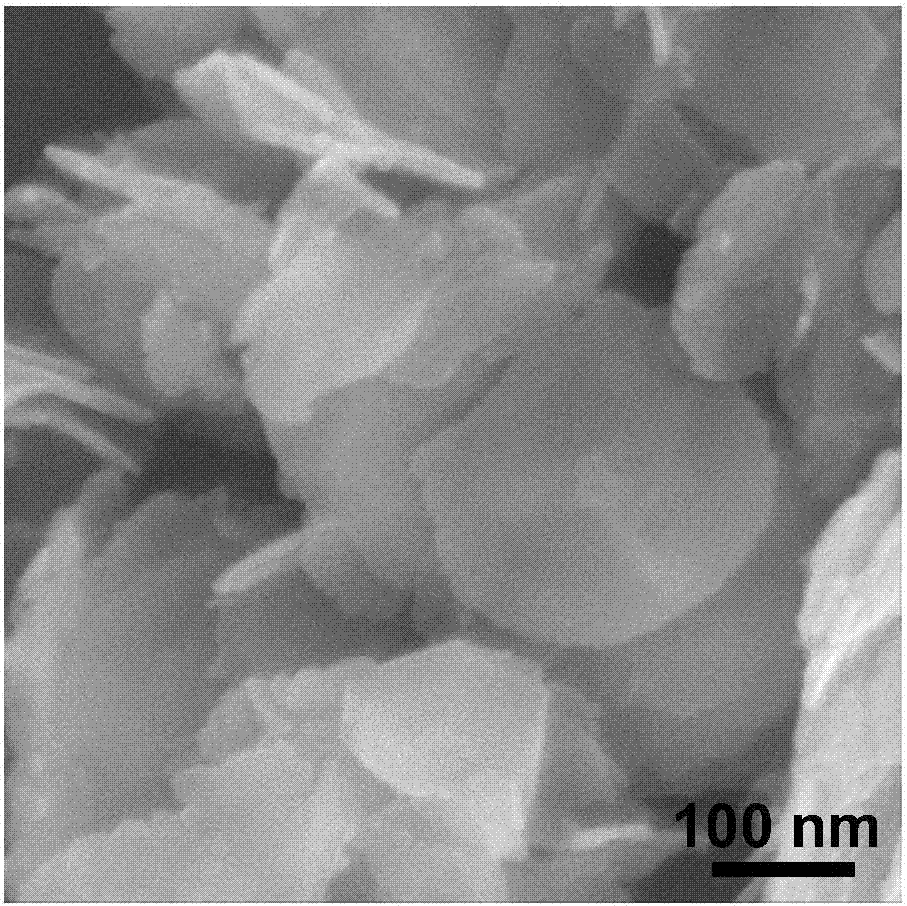
[0035] The prepared two-dimensional CuCo was tested by EDS, XRD and element mapping 2 S 4 Nanosheet components were analyzed (e.g. figure 1 , 2 , 5(A), 5(B), 5(C), 5(D)). figure 1 The peaks of C and O come from the organic capping reagents and air adsorbed on the surface, and the rest are peaks of three elements of Cu, Co, and S, and their atomic number ratio is close to 1:2:4, indicating that the ...
Embodiment 2 2
[0037] Example 2 Two-dimensional CuCo 2 S 4 Preparation of nanosheets
[0038] At room temperature, in a clean, dry 250mL three-necked flask, add 1.3g Cu(acac) 2 and 2.5g Co(acac) 2 Solid, 25mL DDA, ultrasonic dispersion, the above mixture at 5 ℃ min -1 Heat up to 120°C at a heating rate of 30 minutes, add 20 mL of DDT, continue to heat up to 245°C at a heating rate of 5°C min-1, stop at this temperature for 30 minutes, cool naturally to room temperature, and centrifuge , washed to obtain two-dimensional CuCo 2 S 4 Nanosheets.
[0039] EDS, XRD and TEM were used to characterize the obtained product, and the obtained product was still CuCo 2 S 4 Nanosheets.
Embodiment 3
[0040] Example 3 Two-dimensional CuCo 2 S 4 Application of nanosheets as electrocatalysts in ORR and OER
[0041] 2D CuCo 2 S 4 The test method of nanosheets as electrocatalysts to catalyze ORR and OER is as follows: Weigh 2.5 mg of CuCo 2 S 4 Nanosheets, dissolved in a mixed solution of 0.5mL water, 0.5mL ethanol and 40μL naphthol, the concentration of the solution is 2.5mgmL -1 , after the ultrasonic dispersion is uniform, take 8 μL of the above solution, and drop it on a clean rotating disc glassy carbon electrode, N 2 After drying under the sun, repeat the above operation once, and it can be used for electrochemical test after drying.
[0042] For an ORR response, first at N 2 Cyclic voltammetry was performed in saturated 0.1M KOH solution. After it stabilized, the gas was replaced with O 2 , into the electrolyte, the cyclic voltammetry test is also performed, and the polarization curves at different rotational speeds are measured after it stabilizes.
[0043] Fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com