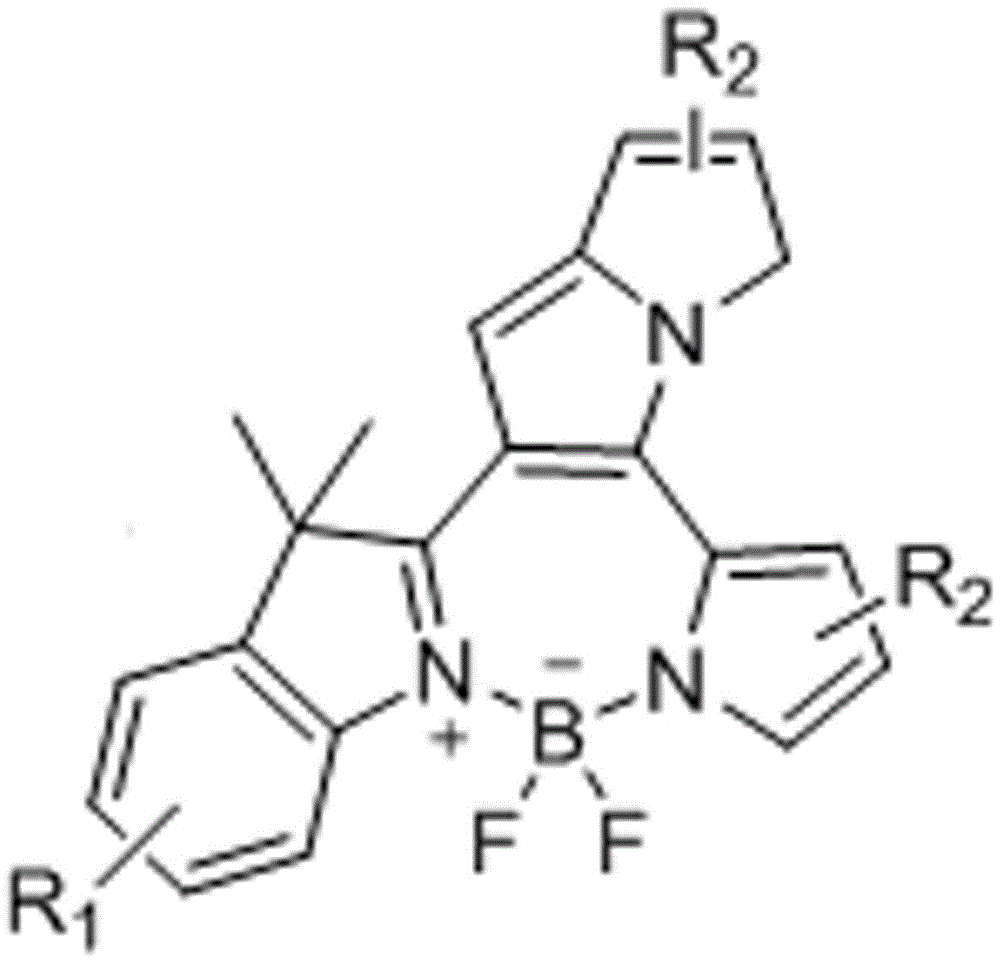
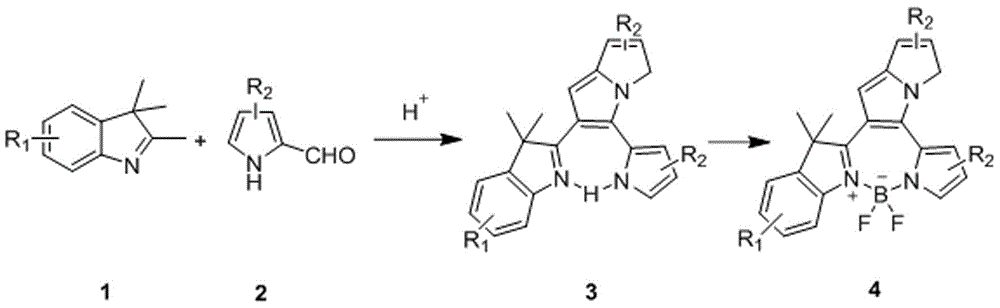
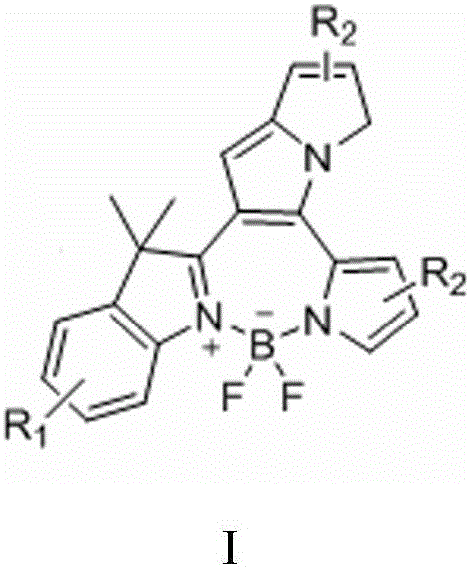
Fluorine-boron pyrrolizinone fluorochrome and synthesizing method thereof
A fluoroboron pyrrolizine, fluorescent dye technology, applied in the direction of azo dyes, organic dyes, luminescent materials, etc., can solve the problems of small Stokes shift, application limitations, etc., achieves few steps, high yield, wide range The effect of research and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Take 1.44g (9.00mmol) of 2,3,3-trimethylindole 1, weigh 0.86g (9.00mmol) of 2-formylpyrrole, measure 0.15L of toluene solution, take 0.24mL of organic acid, and 0.30mL of organic base The mL was mixed, heated and stirred, refluxed at 120°C for 2 hours, spin-dried and purified by filtration to obtain yellow compound 3. Take 1.20g (5mmol) of the above compound 3, take 35mL of toluene solution, 1.00mL of triethylamine, stir under electromagnetic stirring, and add 0.75mL (6.25mmol) of boron trifluoride ether solution dropwise to 120°C, condense and reflux for 0.5 Hours, TLC plate detection, the product was washed, extracted, dried, rotary evaporated, filtered and purified to obtain compound 4:
[0032]
Embodiment 2
[0034] Take 1.44g (9.00mmol) of 2,3,3-trimethylindole 1, weigh 3.44g (36.00mmol) of 2-formylpyrrole, measure 0.15L of toluene solution, take 0.24mL of organic acid, 0.30mL of organic base The mL was mixed, heated and stirred, refluxed at 120°C for 8 hours, spin-dried and purified by filtration to obtain yellow compound 3. Take 1.20g (5mmol) of the above compound 3, take 35mL of toluene solution, 1.00mL of triethylamine, stir under electromagnetic stirring, and add 0.75mL (6.25mmol) of boron trifluoride ether solution dropwise to 120°C, condense and reflux for 0.5 Hours, TLC plate detection, the product was washed, extracted, dried, rotary evaporated, filtered and purified to obtain compound 4:
[0035]
Embodiment 3
[0037] Take 1.44g (9.00mmol) of 2,3,3-trimethylindole 1, weigh 28.50g (0.30mmol) of 2-formylpyrrole, measure 0.15L of toluene solution, take 0.24mL of organic acid, 0.30mL of organic base The mL was mixed, heated and stirred, refluxed at 120°C for 8 hours, spin-dried and purified by filtration to obtain yellow compound 3. Take 1.20g (5mmol) of the above compound 3, take 35mL toluene solution, 1.00mL triethylamine, stir under electromagnetic stirring, and add 0.75mL (6.25mmol) of boron trifluoride ether solution dropwise to 120°C, condense and reflux for 0.5 Hours, TLC plate detection, the product was washed, extracted, dried, rotary evaporated, filtered and purified to obtain compound 4:
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com