Halogen-free expanding flame retardant containing polymeric macromolecule triazine rings and preparation method of halogen-free expanding flame retardant
A technology of intumescent flame retardant and triazine ring, applied in the field of halogen-free intumescent flame retardant containing polymeric macromolecular triazine ring and its preparation, and can solve the problems of great harm to the environment and human body, a large number of them, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
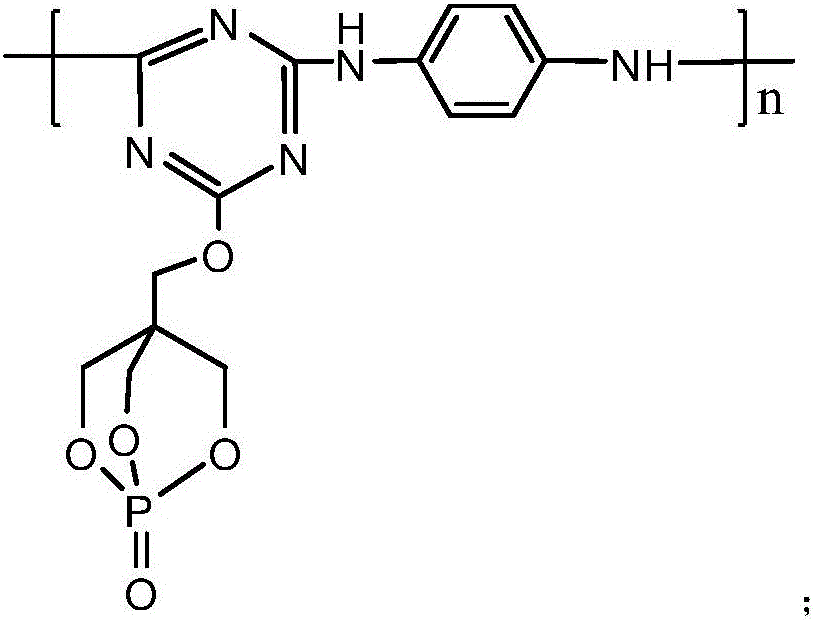
[0036] Add 18.45g of cyanuric chloride and 100ml of acetone solution (the volume ratio of acetone and water is 1:1) in the 1L reaction flask equipped with reflux condenser, constant pressure dropping funnel, stirrer and thermometer, fully stir, Make the cyanuric chloride disperse evenly, then under the condition of 0℃, add 18g of 1-oxyphospha-4-hydroxymethyl-2,6,7-trioxabicyclo[2.2. 2] Octane and NaHCO 3 solution (8.4g NaHCO 3 dissolved in 100ml distilled water), after the dropwise addition was completed, the reaction was carried out at 0° C. for 3 hours. Then raise the temperature to 40°C, add 100ml of acetone solution, and then evenly and slowly add 5.4g of p-phenylenediamine and NaHCO 3 solution (8.4g NaHCO 3 Dissolve in 100ml distilled water), keep the temperature at 40°C, after the dropwise addition is complete, react under this condition for 8 hours. Then suction filter and wash with water to get the reaction product of the second step, put it into a 1L three-neck fl...
Embodiment 2
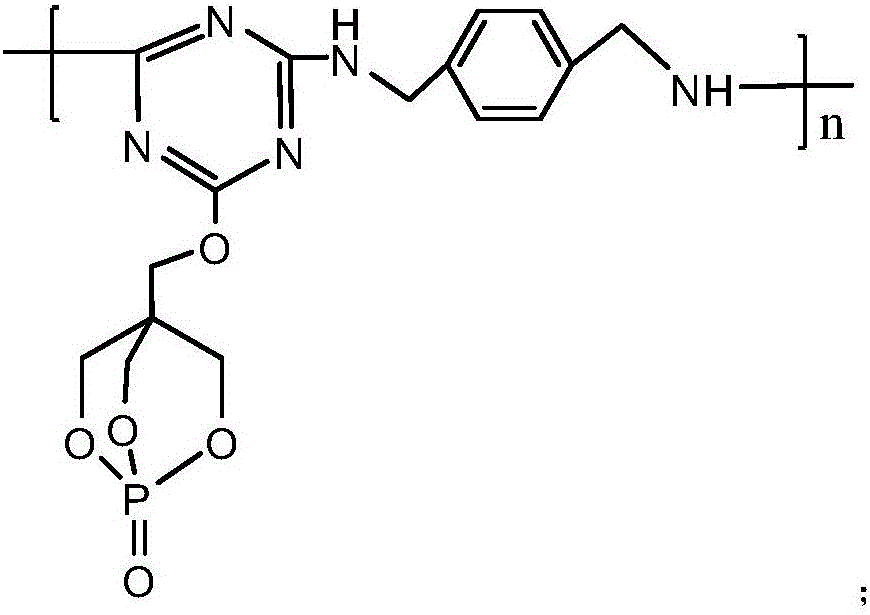
[0040] Add 18.45g of cyanuric chloride and 100ml of acetone solution (the volume ratio of acetone and water is 1:1.5) in the 1L reaction flask equipped with reflux condenser, constant pressure dropping funnel, stirrer and thermometer, fully stir, Disperse cyanuric chloride evenly, and then add 18g of 1-oxyphospha-4-hydroxymethyl-2,6,7-trioxabicyclo[2.2 .2] Octane and NaHCO 3 solution (8.4g NaHCO 3 dissolved in 100ml of distilled water), after the dropwise addition was completed, the reaction was carried out at -5°C for 4 hours. Then raise the temperature to 50°C, add 100ml of acetone solution, and then evenly and slowly add 6.8g of p-xylylenediamine and NaHCO 3 solution (8.4g NaHCO 3 Dissolve in 100ml distilled water), keep the temperature at 50°C, after the dropwise addition is complete, react under this condition for 6 hours. Then suction filter and wash with water to obtain the reaction product of the second step, put it into a 1L three-necked flask, add 300ml of toluen...
Embodiment 3
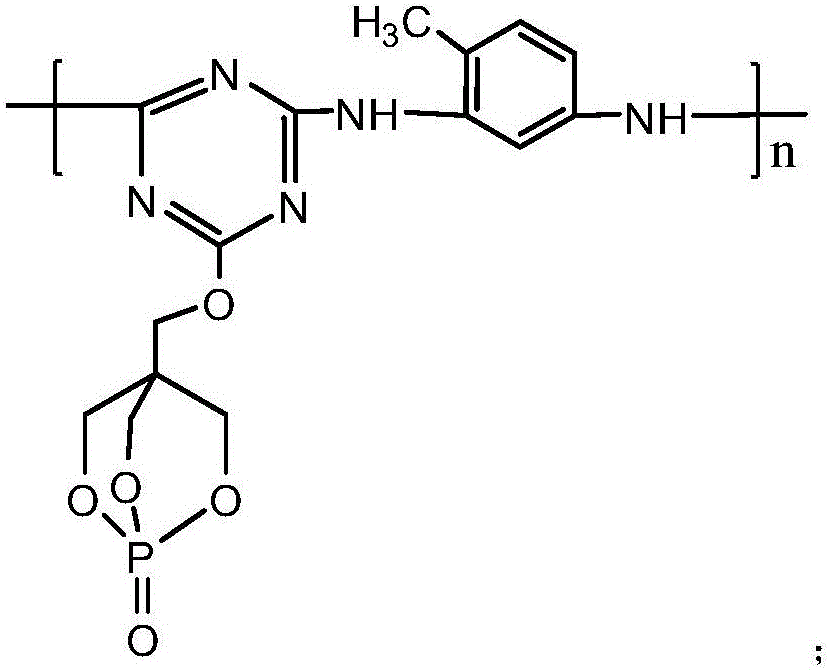
[0044] Add 18.45g of cyanuric chloride and 100ml of acetone solution (the volume ratio of acetone and water is 1:1) in the 1L reaction flask equipped with reflux condenser, constant pressure dropping funnel, stirrer and thermometer, fully stir, Make the cyanuric chloride disperse evenly, then under the condition of 5℃, add 18g of 1-oxyphospha-4-hydroxymethyl-2,6,7-trioxabicyclo[2.2. 2] Octane and NaHCO 3 solution (8.4g NaHCO 3 Dissolved in 100ml distilled water), after the dropwise addition was completed, reacted at 5°C for 3 hours. Then raise the temperature to 45°C, add 100ml of acetone solution, and then evenly and slowly add 6.15g of 2,4-diaminotoluene and NaHCO 3 solution (8.4g NaHCO 3 Dissolve in 100ml distilled water), keep the temperature at 45°C, after the dropwise addition is complete, react under this condition for 7 hours. Then suction filter and wash with water to obtain the reaction product of the second step, put it into a 1L three-necked flask, add 300ml of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com