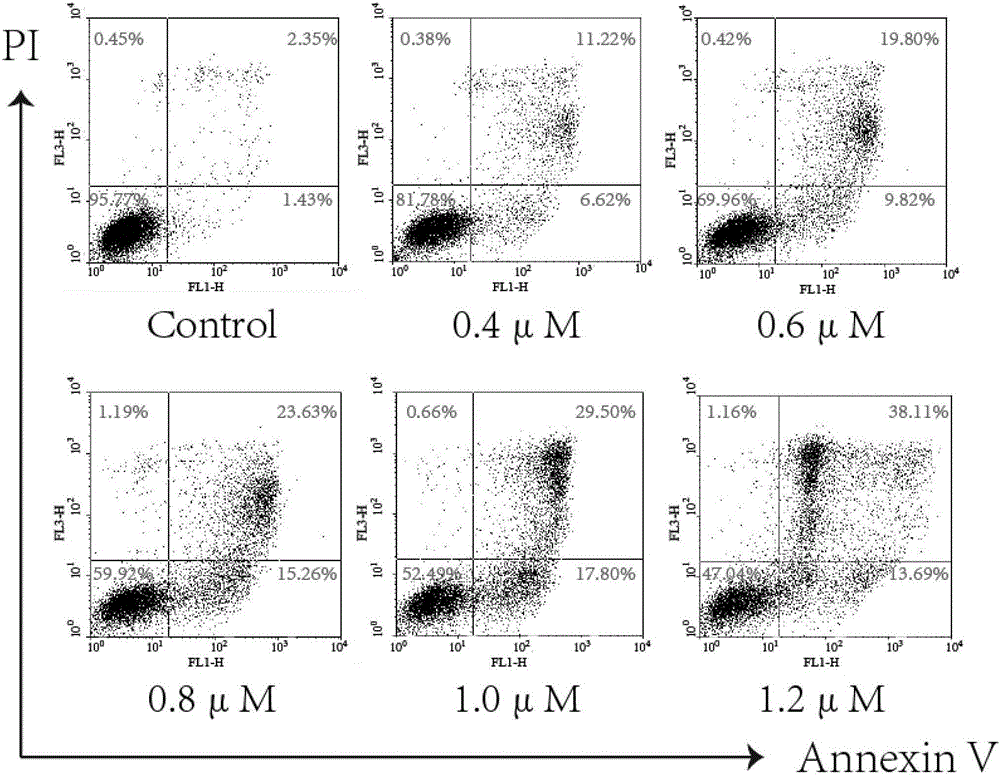
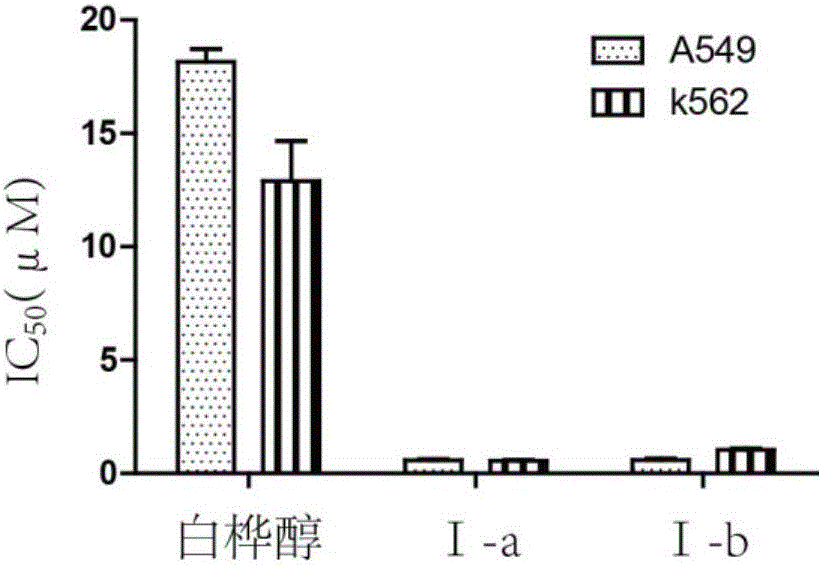
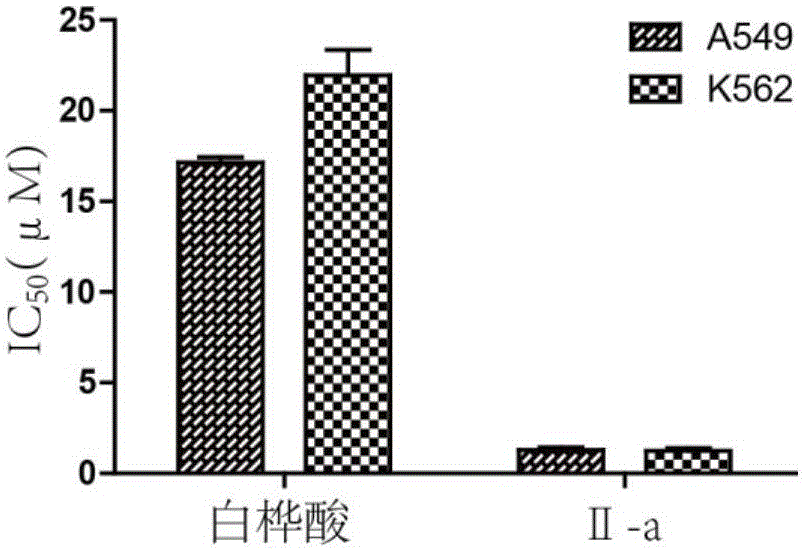
Mitochondria-targeted anti-tumor pentacyclic triterpene derivatives, preparation method and application thereof
A technology of pentacyclic triterpenes and derivatives, which is applied in the field of medicine, can solve problems such as in-depth research, synthesis methods of tumor cell apoptosis, and complexity, and achieve the effects of stable process, strong operability, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: Compound Ⅰ-a preparation
[0061] Betulin (1.0g, 2.26mmol) was mixed with EDC (1.09g, 9.04mmol), DMAP (112mg, 0.90mmol), 5-bromovaleric acid (1.68g, 9.04mmol) and placed in a 100ml round bottom flask, and added 40ml of dichloromethane, reacted at room temperature for about 11h, TLC detected that the reaction reached equilibrium, added triple distilled water and washed three times, and the organic phase was dried with anhydrous sodium sulfate for 7h, then filtered to remove sodium sulfate, concentrated under reduced pressure, silica gel column chromatography to obtain compound 1 250mg of white powder, dissolve this white powder in 10ml of acetonitrile, add 325mg (1.24mmol) of TPP, heat up the oil bath to 75-80°C, stir magnetically for 24 hours, and TLC detects that the reaction is basically complete. After the solvent is evaporated to dryness under reduced pressure, the silica gel column Chromatography yielded 30 mg of the product as white crystals I-a. Da...
Embodiment 2
[0064] Embodiment 2: Compound I-b preparation
[0065] The operation was the same as that of compound Ⅰ-a to obtain a white solid. Data forⅠ-b: Mp: 145-154℃[α] D 20 =+23.2°(c0.1, CH 3 OH), 1 H NMR (CD 3 OD 400MHz,)7.94-7.88(m,6H)7.87-7.76(m,24H)4.73(s,1H),4.61(s,2H),4.44(d,J=5.1Hz,1H),4.39(d, J=10.4Hz, 1H), 3.83(d, J=11.0Hz, 1H), 3.55–3.44(m,5H), 2.49–2.38(m,5H), 1.97–1.84(m,5H).1.72 1.071. 03 0.89 0.83 0.81(s,3H)(6-CH3). 13 C NMR (CD 3 OD,100MHz):173.59,173.10,149.98,134.95,133.45,130.20,118.90,118.04,109.26,81.04,63.89,62.20,55.31,50.22,48.54,48.18,47.86,47.60,47.22,47.20,47.10,47.00,46.36 , 42.45, 41.85, 40.71, 38.09, 37.54, 36.84(s), 34.18, 33.91, 33.17, 32.77, 29.90, 29.34, 28.90, 27.19, 26.80, 25.54, 25.08, 23.35, 23.06, 23.79, 20.79, 21.06 20.54, 17.94, 15.71, 15.28, 13.87, 13.10, 10.08; HR-ESI-MS (m / z): [(M-2Br) / 2] + .
[0066] The synthetic route is as follows:
[0067]
Embodiment 3
[0068] Embodiment 3: Compound I-c preparation
[0069] Betulin (5.0g, 11.3mmol) was mixed with EDC (5.45g, 45.2mmol), DMAP (560mg, 4.5mmol), 5-bromovaleric acid (8.4g, 45.2mmol) and placed in a 500ml round bottom flask, and added 200ml of dichloromethane, reacted at room temperature for about 11h, TLC detected that the reaction reached equilibrium, added triple steamed water and washed three times, and the organic phase was dried with anhydrous sodium sulfate for 7h, then filtered to remove sodium sulfate, concentrated under reduced pressure, silica gel column chromatography to obtain compound 1 Take 1.19 g of the above-mentioned compound 1 and dissolve it in 20 mL of formic acid, raise the temperature of the oil bath to 85 ° C and stir for 2 h, and then TLC detects that the reaction is basically complete. After drying with sodium sulfate in water for 7 hours, remove the sodium sulfate by suction filtration, concentrate under reduced pressure, and obtain 700 mg of compound 3 b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com