Method for producing cyclobutane tetracarboxylic acid derivative
A technology of cyclobutane tetracarboxylic acid and its manufacturing method, which can be used in electrical components, circuits, organic chemistry, etc., and can solve the problems of unrecorded isomers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0078] Hereinafter, although an Example is given and this invention is demonstrated concretely, this invention is not limited to these Examples.
[0079] In addition, the analysis method used in the Example is as follows.
[0080]
[0081] Device: GC-2010Plus (manufactured by Shimadzu Corporation),
[0082] Column: DB-1 (manufactured by GL Sciences Inc.) 0.25 mm×30 m, film thickness 0.25 μm,
[0083] Carrier gas: He, detector: FID, sample injection volume: 1μL, injection port temperature: 160°C, detector temperature: 220°C, column temperature: 70°C (20min)-40°C / min-220°C (15min) , Split ratio: 1:50, internal standard substance: butyl lactate.
[0084] 1H NMR analysis conditions>
[0085] Apparatus: Fourier transform type superconducting nuclear magnetic resonance apparatus (FT-NMR) INOVA-400 (manufactured by Varian Corporation) 400MHz,
[0086] Solvent: DMSO-d6, internal standard substance: tetramethylsilane (TMS).
[0087]
[0088] Device: DSC1 (manufactured by Mettl...
Embodiment 1
[0096]
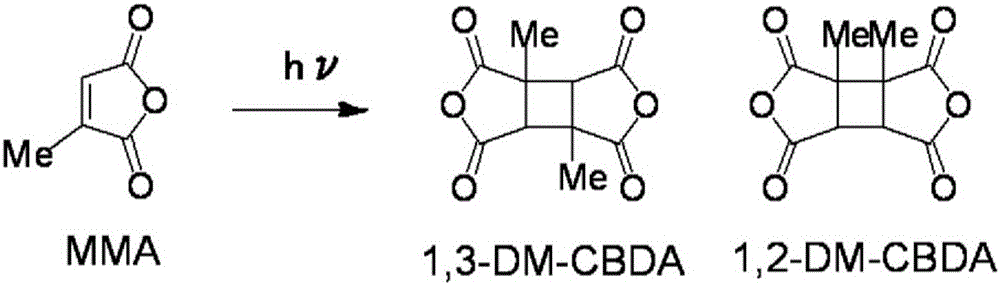
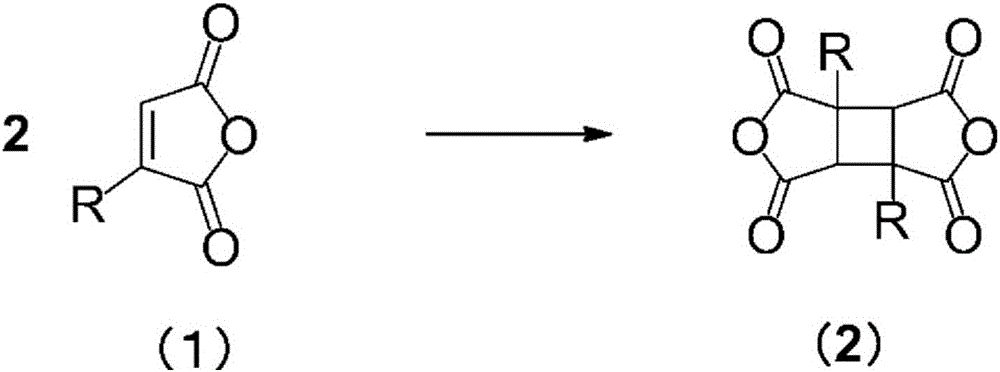
[0097] In a nitrogen atmosphere, 0.10 g (0.89 mmol) of citraconic anhydride (CA) and 20 g (222 mmol) of dimethyl carbonate (200 wt. ), stirred with a magnetic stirrer to dissolve it. Then, stirring at 15-20 degreeC, it irradiated with the 100W high-pressure mercury lamp for 4 hours. As a result of quantitative analysis of the reaction solution by gas chromatography after irradiation, the residual rate of citraconic anhydride (CA) was 26.2%. In addition, 2 g of the reaction liquid in the reactor was collected, and the solvent was distilled off at 70-80 Torr with an evaporator. pass 1 It was confirmed by H NMR analysis that the obtained crude product was a mixture containing 1,3-DM-CBDA and 1,2-DM-CBDA (1,3-DM-CBDA:1,2-DM-CBDA=48.3:51.7).
Embodiment 3
[0105]
[0106] Under a nitrogen atmosphere, 35.0 g (312 mmol) of citraconic anhydride (CA) and 152 g (1682 mmol, 4.33 wt. ), stirred with a magnetic stirrer to dissolve it. Then, stirring at 10-15 degreeC, it irradiated with the 100W high-pressure mercury lamp for 48 hours. It was confirmed by gas chromatographic analysis that the residual ratio of raw materials in the reaction liquid was 23.7%. Next, the precipitated white crystals were taken out by filtration at 10-15°C, and the crystals were washed twice with 43.8 g (497 mmol, 1.25 wt times relative to citraconic anhydride (CA)) of ethyl acetate. This was dried under reduced pressure to obtain 8.1 g of white crystals (yield 23.1%). pass 1 It was confirmed by H NMR analysis that the crystal was a mixture containing 1,3-DM-CBDA and 1,2-DM-CBDA (1,3-DM-CBDA:1,2-DM-CBDA=90.3:9.7). In addition, the obtained crystals, filtrate, and cleaning solution were respectively used 1 H NMR analysis and quantitative analysis by gas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com