Fluorogenic quantitative PCR detection reagent and preparation method and application thereof
A fluorescent quantitative and detection reagent technology, applied in the field of animal disease quarantine and detection, can solve problems such as economic losses in the poultry industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
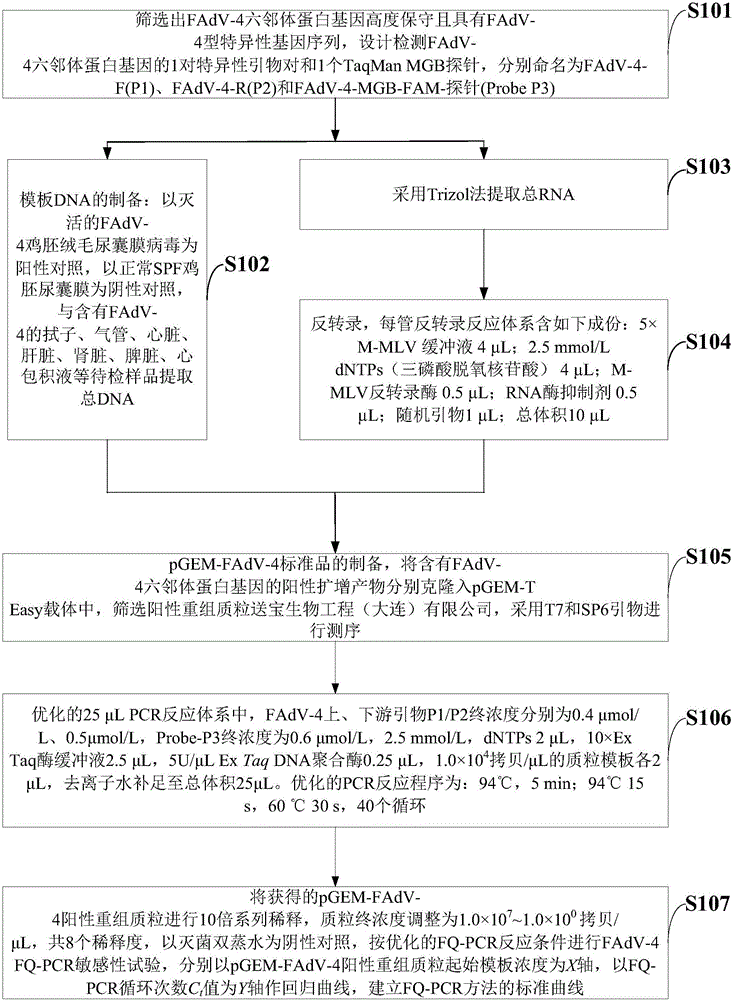
[0041] Such as figure 1 As shown, the preparation method of the fluorescence quantitative PCR detection reagent of the embodiment of the present invention comprises the following steps:
[0042] S101: Screen out the FAdV-4 hexon protein gene which is highly conserved and has FAdV-4 type-specific gene sequence, design a pair of specific primer pairs and a TaqMan MGB probe for detecting the FAdV-4 hexon protein gene, Named respectively as FAdV-4-F(P1), FAdV-4-R(P2) and FAdV-4-MGB-FAM-probe (ProbeP3);
[0043] S102: Preparation of template DNA: with the inactivated FAdV-4 chicken embryo chorioallantoic membrane virus as a positive control, and the normal SPF chicken embryo allantoic membrane as a negative control, the swabs, trachea, heart, Liver, kidney, spleen, and pericardial effusion samples to extract total DNA;
[0044] S103: extracting total RNA by Trizol method;
[0045] S104: Reverse transcription, each tube of reverse transcription reaction system contains the follow...
Embodiment 1
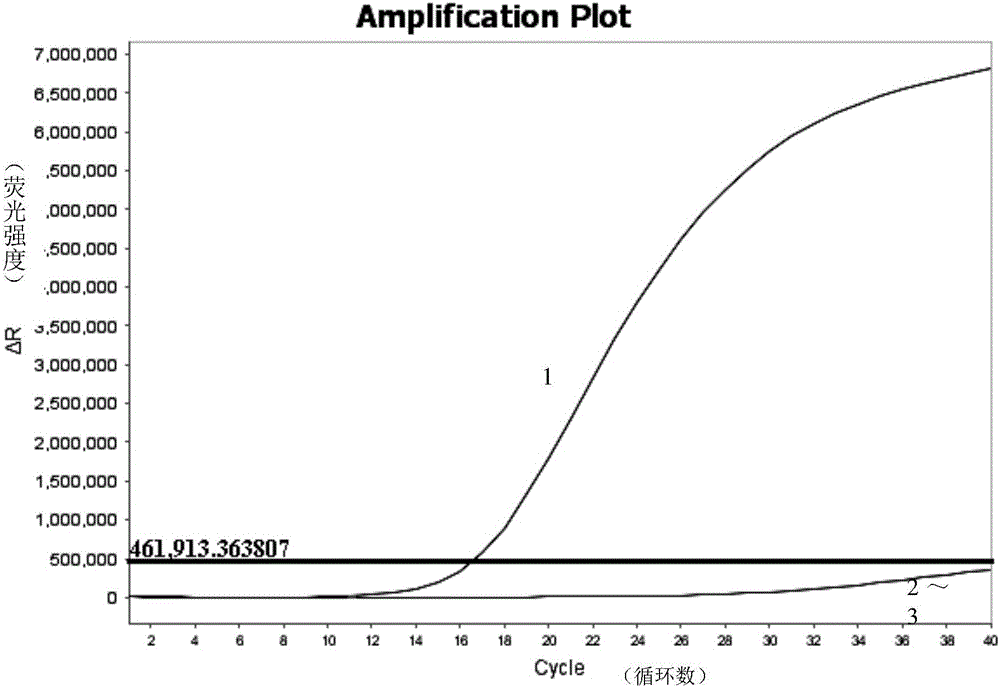
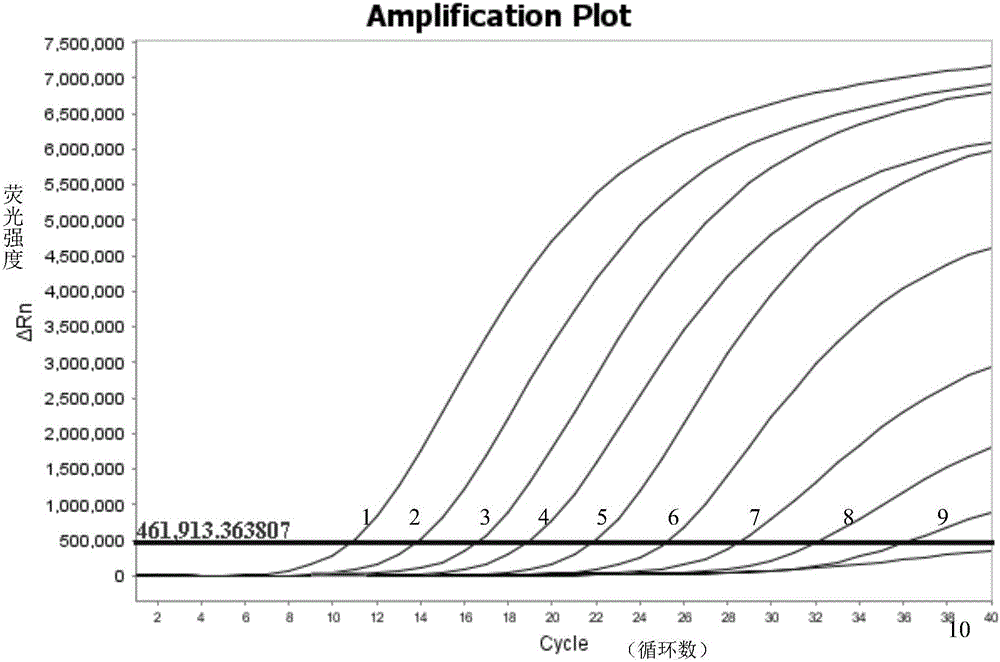
[0050] Embodiment 1, for the establishment of primers and probes and detection methods for the detection of the 4th serotype virus (FAdV-4) TaqMan MGB fluorescence quantitative PCR (FQ-PCR) of avian adenovirus 1 subgroup C
[0051] 1.1 Materials
[0052] 1.1.1 Strains
[0053] The inactivated avian adenovirus subgroup I subgroup C serotype 4 virus (FAdV-4) was isolated and identified by Henan Animal Disease Prevention and Control Center; the other 11 serotype viruses (FAdV-4) 1-3, FAdV-5-12) were purchased from the China Veterinary Drug Control Institute; other control viruses, bacteria or positive recombinant plasmids were provided by the Animal Disease Prevention and Control Center of Henan Province.
[0054] 1.1.2 Instruments and reagents
[0055] Fluorescence PCR instrument, product of American ABI Company, model ABI VⅱA 7; PCR amplification instrument, product of German Biometra Company; gel imaging analysis system, product of American Alpha Innotech Company; constant t...
Embodiment 2
[0093] Example 2, application of TaqMan MGB fluorescence quantitative PCR (FQ-PCR) detection technology for avian adenovirus I subgroup C serotype 4 virus (FAdV-4).
[0094] According to the avian adenovirus I subgroup C kind 4th serotype virus (FAdV-4) FQ-PCR detection method established in Example 1 of the present invention and the PCR method established in this study, prepare detection reagents, with inactivated FAdV-4 Chicken embryo chorioallantoic membrane virus was used as a positive control, and normal SPF chicken embryo allantoic membrane was used as a negative control. 47 samples of clinically suspected FAdV-4 infection (sample number: 1-47) were tested by application. The detection results of the method were compared and analyzed, and compared with the sequencing results. The results showed that 17 samples were positive for FAdV-4 gene by using the FAdV-4 FQ-PCR method provided by the present invention, and the numbers were 8-10, 16, 18, 20, 32-38, 41, 42, 45, 47 res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com