Polyacrylic acid-cystamine dihydrochloride-vitamin E succinate polymer, and preparation method and application thereof
A technology of cystamine dihydrochloride and succinate, applied in the direction of active ingredients of heterocyclic compounds, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of large side effects and low drug bioavailability, and achieve Mild conditions, low critical micelle concentration, improved safety and efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The synthetic method of this polyacrylic acid-cystamine dihydrochloride-vitamin E succinate polymer, comprises the steps:
[0041] (1) Synthesis of cystamine dihydrochloride-vitamin E succinate: dissolve vitamin E succinate in anhydrous N,N-dimethylformamide, add 1-(3-dimethylaminopropyl base)-3-ethylcarbodiimide hydrochloride (EDCI), 1-hydroxybenzotriazole (HOBT), stirred at room temperature for 2-4h, and then added cystamine dihydrochloride, triethyl Amine, under room temperature, avoid light and stir for 12 hours. After the reaction is completed, add the reaction solution dropwise into a beaker containing ice water under the condition of stirring. After the addition, a white solid precipitates out, which is filtered and freeze-dried to obtain cystamine di Hydrochloride - Vitamin E succinate product.
[0042] (2) Synthesis of polyacrylic acid-cystamine dihydrochloride-vitamin E succinate: dissolve polyacrylic acid in anhydrous N,N-dimethylformamide, add 1-(3-dimethyl...
Embodiment 1
[0051] Embodiment 1. the synthesis of cystamine dihydrochloride-vitamin E succinate
[0052] 1) Dissolve vitamin E succinate (VES, 0.53g, 1mmol) in 20mL of anhydrous N,N-dimethylformamide, put it in a 50mL round bottom flask, and after dissolving, add 1-(3 -Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 0.23g, 1.2mmol), 1-hydroxybenzotriazole (HOBT, 0.18g, 1.2mmol), stirring at room temperature React for 2-4h, then add cystamine dihydrochloride (0.675g, 3mmol), 1mL triethylamine, and stir for 12h at room temperature in the dark. Add it into a beaker containing 200mL of ice water. After the addition, a white solid precipitates out. After filtering and freeze-drying, the cystamine dihydrochloride-vitamin E succinate solution is obtained.
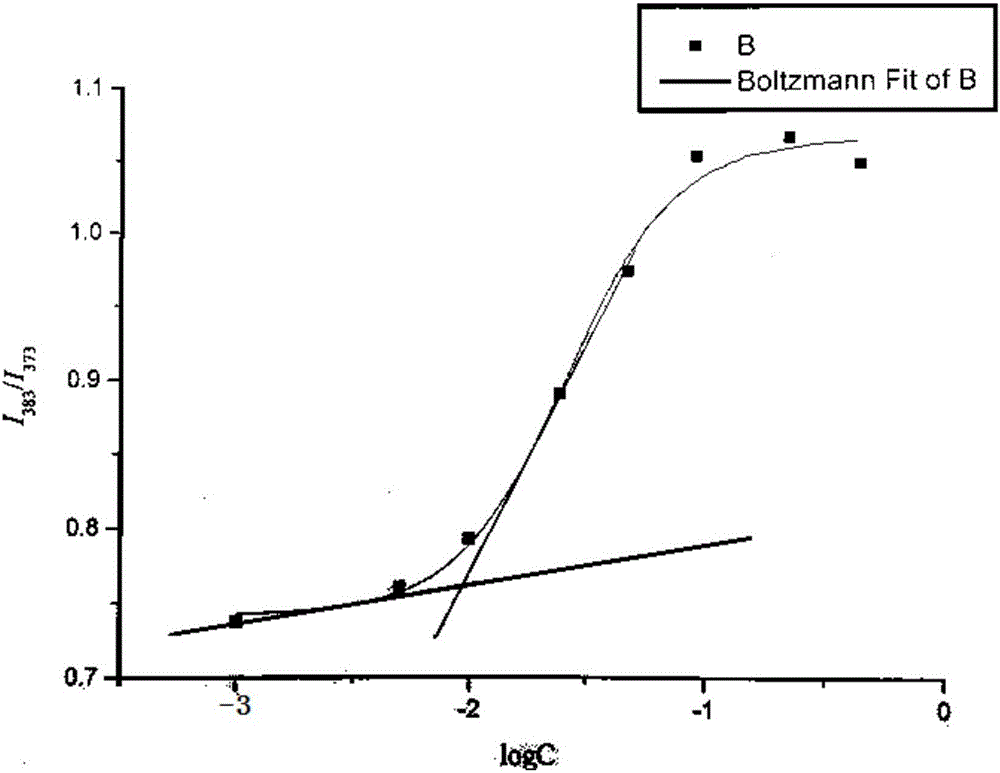
[0053] The structure of the compound in Example 1 is determined by proton nuclear magnetic resonance spectroscopy, and the solvent is CDCl 3 , the result is as figure 1 shown. The chemical shift is 6.5ppm for the H in -NH-CO...
Embodiment 2
[0054] Embodiment 2. the synthesis of polyacrylic acid-cystamine dihydrochloride-vitamin E succinate
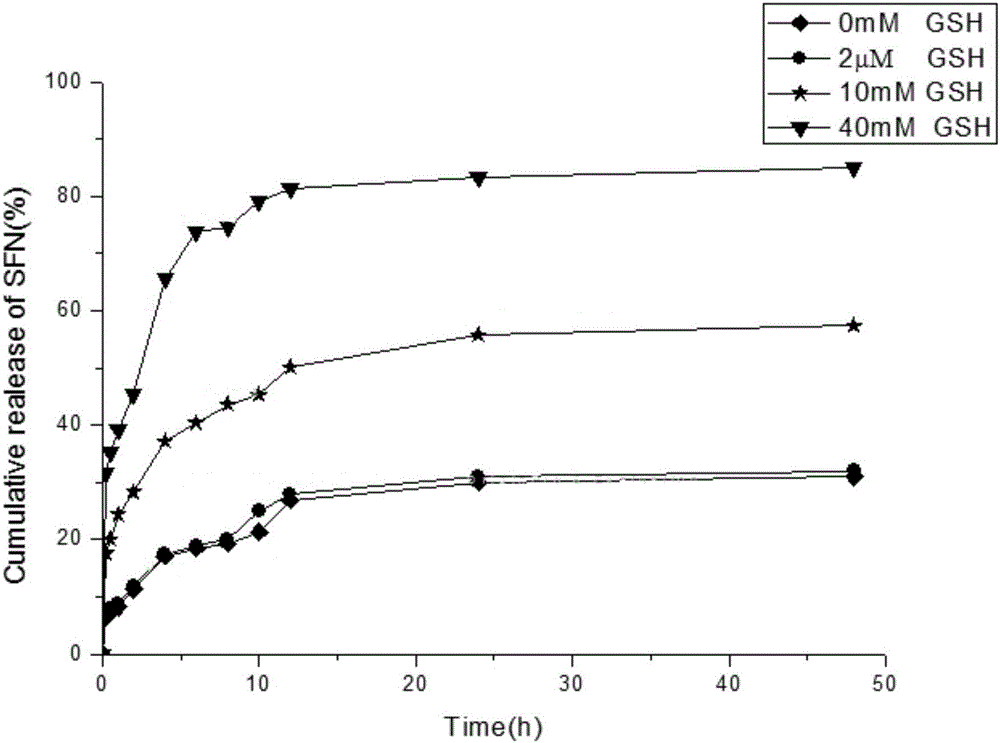
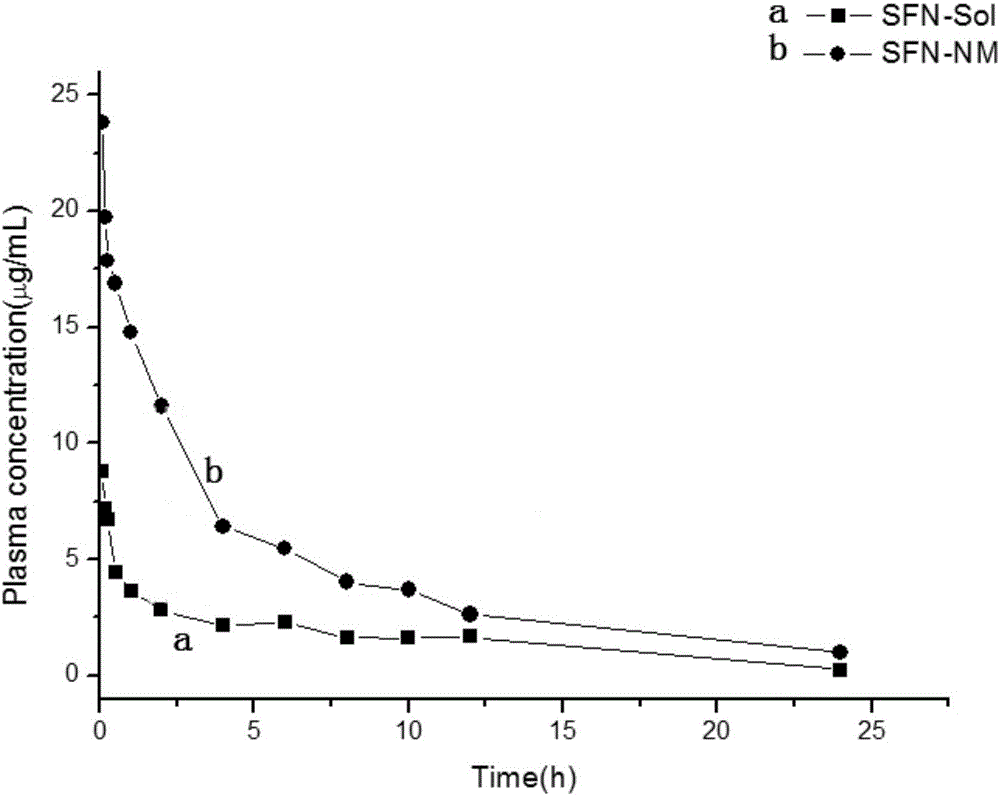
[0055] 2) Dissolve polyacrylic acid (0.18g, 2.5mmol) in 20mL of anhydrous N,N-dimethylformamide, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiethylene Amine hydrochloride (EDCI, 0.115g, 0.6mmol), 1-hydroxybenzotriazole (HOBT, 0.09g, 0.6mmol), stirred and reacted at room temperature for 6h to obtain a mixed solution, and then added 5mL to the mixed solution dropwise A solution of cystamine dihydrochloride-vitamin E succinate (0.332 g, 0.5 mmol) in N,N-dimethylformamide was stirred and reacted at room temperature for 12 h in the dark. After the reaction, the reaction solution was transferred to a dialysis bag, dialyzed at room temperature for 3-5 days, and freeze-dried to obtain a white powder, that is, polyacrylic acid-cystamine dihydrochloride-vitamin E succinate.
[0056] Determination by NMR 1 HNMR determines the combination of compound in embodiment 2, adopts DMSO-d6 as s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com