Analytical method of lithium manganate series adsorbent precursors
An analytical method and adsorbent technology, applied in the direction of manganate/permanganate, etc., can solve the problems of large consumption of ammonium persulfate, poor efficiency, and short service life of the membrane, so as to increase the concentration of lithium ions and inhibit the dissolution Loss, the effect of prolonging the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
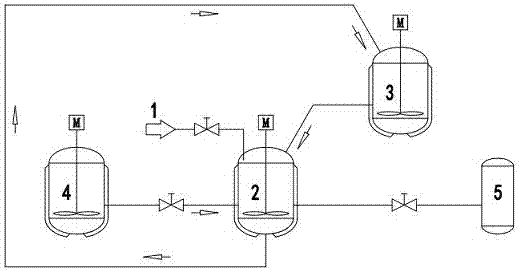
[0022] Such as figure 1 As shown, the brine used is the old brine provided by Qinghai CITIC Guoan (Mg 2+ :118.3g / L, Li + : 4.382g / L, adjust pH to 5.5 in advance), adsorbent precursor Li 1.33 mn 1.67 o 4 According to the relevant literature synthesis (J.Mater.Chem., 1999, 9, 2683-2690). Take by weighing 1 kilogram of adsorbent precursor, 0.3 kilogram of inhibitor lead dioxide, the two are fully mixed, placed in the adsorption analysis pool 2, add 1.6 liters of 1.85mol / L sulfuric acid solution (H + ) was analyzed for 15 minutes, filtered, the filtrate was discarded, and 1.6 liters of 1.85mol / L sulfuric acid solution (H + ) analysis for 15 minutes, filtered, and the filtrate was input into the storage pool 3 for standby; after the adsorbent obtained from the initial analysis was washed, 4L of old brine was added to the adsorption analysis pool 2 through the brine pipeline 1, stirred and adsorbed at room temperature for 30 minutes to obtain the adsorbent precursor, filtered, ...
Embodiment 2
[0024] Such as figure 1 As shown, the brine used is the old brine provided by Qinghai CITIC Guoan (Mg 2+ :118.3g / L, Li + : 4.382g / L, adjust pH to 5.5 in advance), adsorbent precursor Li 4 mn 5 o 12 Synthesized according to relevant literature (J. Electrochem. Soc., 1998, 115, 153-155). Take by weighing 2 kilograms of adsorbent precursors, 0.5 kilograms of inhibitor lead dioxide, the two are fully mixed, placed in the adsorption analysis pool 2, add 3.5 liters of 2.33mol / L sulfuric acid solution (H + ) was analyzed for 15 minutes to obtain the adsorbent precursor, filtered, and the filtrate was discarded, and 3.5 liters of 2.33 mol / L sulfuric acid solution (H + ) for 15 minutes, filtered, and the filtrate was input into the storage pool 3 for standby; after the adsorbent obtained for the first time was washed, 7 L of old brine was added through the brine pipeline 1, stirred and adsorbed at room temperature for 30 minutes to obtain the adsorbent precursor, filtered, washed,...
Embodiment 3
[0026] Such as figure 1 As shown, the brine used is the old brine provided by Qinghai CITIC Guoan (Mg 2+ :118.3g / L, Li + : 4.382g / L, adjust pH to 5.5 in advance), adsorbent precursor LiMn 2 o 4purchased by the market. Take by weighing 2 kilograms of adsorbent precursors, 0.6 kilograms of inhibitor lead dioxide, the two are fully mixed, placed in the adsorption analysis pool 2, add 3.5 liters of 2.05mol / L sulfuric acid solution (H + ) for 15 minutes, filtered, discarded the filtrate, and added 3.5 liters of 2.05 mol / L sulfuric acid solution (H + ) for 15 minutes, filtered, and the filtrate was input into the storage pool 3 for standby; the adsorbent obtained for the first time was washed and added to 7 L of old brine, stirred and adsorbed at room temperature for 30 minutes, filtered, washed, and added to the second round of the previous round through the storage pool 3 Analyze the solution, analyze it for 10 min under stirring at room temperature, filter, and 3.32 L of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com