Preparation method of hexagonal wurtzite structure copper-zinc-tin-sulfur nano-crystal
A hexagonal wurtzite, copper-zinc-tin-sulfur technology, applied in the direction of nanotechnology, nanotechnology, chemical instruments and methods, can solve the problems of use, environmental pollution, resource waste, etc., and achieve low cost, easy promotion, and small size Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
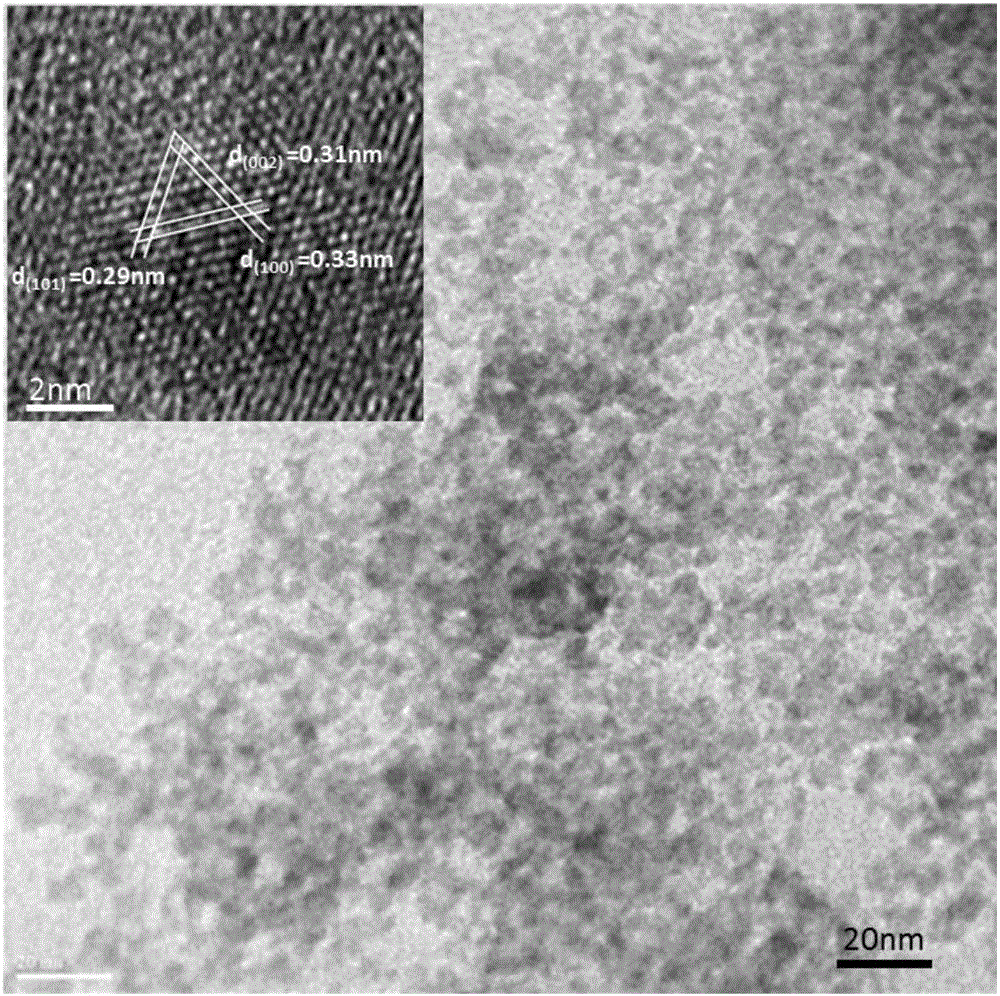
[0028] In this example, hexagonal wurtzite structure copper-zinc-tin-sulfur nanocrystals were prepared according to the following steps:
[0029] a. Take 0.375mmol Cu 2 O nanocubic particles were dispersed in 10 mL of deionized water to form Cu 2 O nano cubic particle dispersion;
[0030] b. Dissolve 5mL of SnCl with a concentration of 0.075M 4 The solution was added dropwise to 10mL of 0.3M Na 2 In the aqueous solution of S, stir to form a colorless and transparent solution B;
[0031] c, the Cu obtained in step a 2 The O nanocubic particle dispersion liquid adds in the solution B that step b obtains, and adding 5mL concentration is 0.075M Zn(NO 3 ) 2 solution, adjust the pH value to 7, and stir for 10 minutes to obtain a mixed solution;
[0032] d. Add the mixed solution into the reaction kettle and react at 190°C for 24 hours; after cooling the reaction kettle to room temperature, centrifuge to obtain the precipitate, wash it with absolute ethanol for several times a...
Embodiment 2
[0039] Na in test step b 2 The effect of the amount of S on the purity of the product, this example prepares the hexagonal wurtzite structure CZTS nanocrystals according to the same method as in Example 1, the difference is only that the Na in step b 2 The amount of S solution was changed to 10mL, 0.15M.
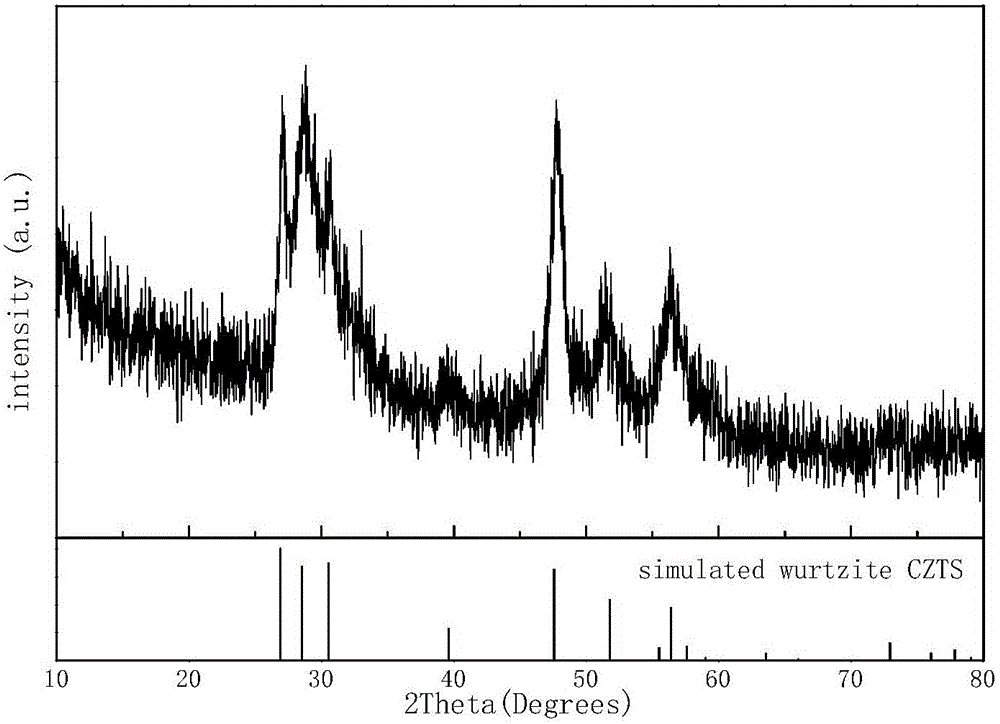
[0040] The X-ray diffraction pattern of the obtained sample is as Figure 6 As shown, it can be seen from the figure that there are many impurity peaks (marked with an asterisk in the figure).
Embodiment 3
[0042] In order to test the effect of the pH value on the crystal phase of the product in step c, this example prepared hexagonal wurtzite structure CZTS nanocrystals according to the same method as in Example 1, the only difference being that the pH value in step c was adjusted to 7 and 8 in sequence , 9, 10, 11, 12.
[0043] The X-ray diffraction pattern of each sample obtained is as follows Figure 7 As shown, it can be seen from the figure that as the pH value decreases, the product gradually transforms from a tetragonal kesterite structure to a hexagonal wurtzite structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com