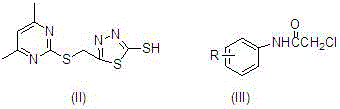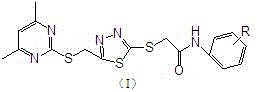Thioether compounds containing pyrimidine, thiadiazole ring and amide structures and application thereof
A technology of thiadiazole rings and compounds, which is applied in the field of thioether compounds, can solve the problems of structure and biological activity that have not been reported in literature, and achieve the effect of simple preparation and good antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
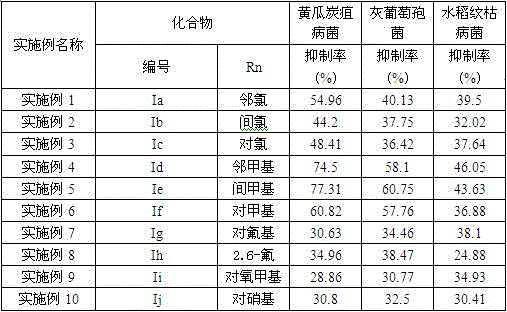
[0030] The synthesis of embodiment 1 derivative Ia (Rn=o-chloro)
[0031] Synthesis of 5-((4,6-dimethylpyrimidin-2-ylthio)methyl)-1,3,4-thiadiazole-2-thiol: In a 250 mL one-necked flask, weigh 2.60 g KOH (0.045 mol) was slowly added into 100 mL of absolute ethanol with stirring until dissolved, and 0.03 mol of 2-((4,6-dimethylpyrimidin-2-yl)thio)acetylhydrazide was weighed added to the solution. Under the condition of ice bath, slowly add 0.5 mL CS2 (0.045 mol) dropwise, after the dropwise addition, continue to stir at room temperature for 5 h, then filter with suction, wash with ether, and dry to obtain the intermediate. Under ice-bath conditions, in a 50 mL single-necked flask, add 20 mL of concentrated sulfuric acid, slowly add 0.014mol 2-((4,6-dimethylpyrimidin-2-yl)thio)acetylhydrazinodithio Potassium formate, after all the solids are dissolved, stir at room temperature for 2 h, slowly pour the mixture into ice water and stir, a large amount of white solids will appear ...
Embodiment 2
[0036] Example 2 Synthesis of Derivatives Ib (Rn=m-chloro)
[0037] Dissolve 5 mmol of 5-((4,6-dimethylpyrimidin-2-ylthio)methyl)-1,3,4-thiadiazole-2-thiol in NaOH aqueous solution, then add dissolved 5.5 mmol of N-substituted m-chlorophenyl-2-chloroacetamide in 5 mL of absolute ethanol reacted in an alkaline aqueous solution to precipitate a solid product, and finally obtained a white powder after suction filtration, washing, drying, and ethanol recrystallization Like solid, that is, the product. The melting point is 128°C, and the yield is 83.4%.
[0038] of the compound 1 H NMR and IR spectroscopic data are described below,
[0039] 1 H NMR (CDCl 3 ) δ: 9.83 (s, 1H, -NH), 7.71 (s, 1H, Ar-H), 7.39 (s, 1H, Ar-H), 7.25 – 7.18 (m, 1H, Ar-H), 7.07 ( d, J = 7.4 Hz, 1H, Ar-H), 6.86 (s, 1H, CH), 4.76 (s, 2H, CH 2 ), 4.05 (s, 2H,CH 2 ), 2.52 (s, 6H, (CH 3 ) 2 ) ; IR (KBr)ν:3210, 3063, 2917, 1681, 1626, 1596, 1482, 1272, 1071 cm -1 .
Embodiment 3
[0040] Example 3 Synthesis of Derivatives Ic (Rn=Pair Chlorine)
[0041] Dissolve 5 mmol of 5-((4,6-dimethylpyrimidin-2-ylthio)methyl)-1,3,4-thiadiazole-2-thiol in NaOH aqueous solution, then add dissolved 5.5 mmol of N-substituted p-chlorophenyl-2-chloroacetamide in 5 mL of absolute ethanol reacted in an alkaline aqueous solution to precipitate a solid product, and finally obtained a white powder after suction filtration, washing, drying, and ethanol recrystallization Like solid, that is, the product. The melting point is 135-137°C, and the yield is 91.3%.
[0042] of the compound 1 H NMR and IR spectroscopic data are described below,
[0043] 1 H NMR (CDCl 3 ) δ: 9.82 (s, 1H, -NH), 7.50 (d, J = 8.8 Hz, 2H, Ar-H), 7.25(d, J = 8.8 Hz, 2H, Ar-H), 6.80 (s, 1H , CH), 4.71 (s, 2H,CH 2 ), 4.02 (s, 2H,CH 2 ), 2.45 (s, 6H, (CH 3 ) 2 ) ; IR (KBr)ν: 3201, 3045, 2914, 1692, 1584, 1488, 1269, 1066 cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com