Synergistic pesticidal compositions and related methods
An insecticidal composition and composition technology, applied in synergistic insecticidal compositions and related fields, can solve problems such as not necessarily giving satisfactory insect control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Preparation of 3-((3,3,3-trifluoropropyl)thio)propionyl chloride
[0079]
[0080] A dry five-liter round-bottomed flask equipped with a magnetic stirrer, nitrogen inlet, reflux condenser and thermometer was charged in dichloromethane (CH 2 Cl 2 ) 3-((3,3,3-trifluoropropyl)thio)propionic acid in (3L) (prepared as described in PCT Publication No. WO 2013 / 062981 by Niyaz et al.) (188 g, 883 mmol). Thionyl chloride (525 g, 321 mL, 4.42 mol) was added dropwise over 50 minutes. The reaction mixture was heated to reflux (about 36°C) for two hours and then cooled to room temperature (about 22°C). The resulting mixture was concentrated on a rotary evaporator under vacuum, and then distilled (40 Torr, the product collected at a temperature of about 123°C to about 127°C) to provide the title compound (177.3g, 86%) as a clear colorless liquid ): 1 H NMR(400MHz, CDCl 3 )δ3.20(t,J=7.1Hz,2H), 2.86(t,J=7.1Hz,2H), 2.78–2.67(m,2H), 2.48–2.31(m,2H); 19 F NMR(376MHz, CDCl 3 )δ-66.42, -66.43...
Embodiment 2
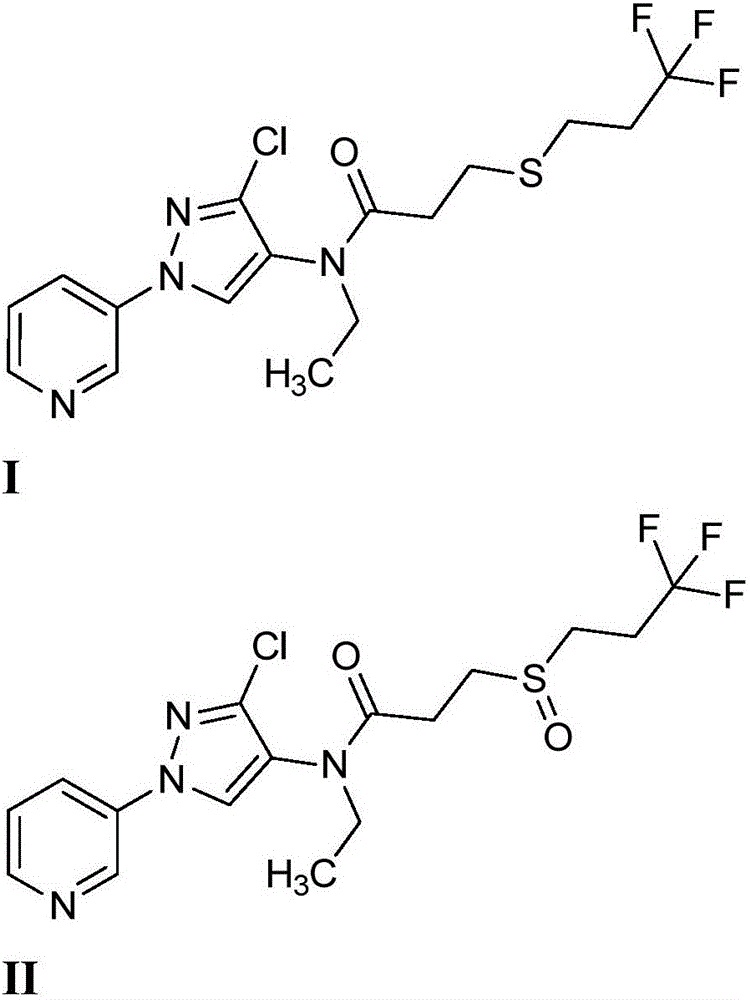
[0082] Preparation of N-(3-chloro-1-(pyridin-3-yl)-1H-pyrazol-4-yl)-N-ethyl-3-((3,3,3-trifluoropropyl)sulfanyl ) Propionamide (I)
[0083]
[0084] At a temperature of about 0℃ and at N 2 Next, add 3-chloro-N-ethyl-1-(pyridin-3-yl)-1H-pyrazol-4-amine (prepared as described in U.S. Publication No. 2012 / 0110702 of Yap et al.) (10.0g , 44.9mmol) in CH 2 Cl 2 (100mL), add pyridine (5.45mL, 67.4mmol), 4-dimethylaminopyridine (DMAP) (2.74g, 22.45mmol) and 3-((3,3,3-trifluoropropyl) sequentially Thio)propionyl chloride (9.91 g, 44.9 mmol). The reaction was warmed to room temperature and stirred for one hour. The reaction mixture was poured into water (100 mL), and the resulting mixture was stirred for five minutes. Transfer the mixture to a separatory funnel and separate the layers. Use CH for water phase 2 Cl 2 (3x 50mL) extraction, the combined organic extracts were subjected to sodium sulfate (Na 2 SO 4 ) Dry, filter, and concentrate in vacuo. The crude product was subjected to ...
Embodiment 3
[0086] Preparation of N-(3-chloro-1-(pyridin-3-yl)-1H-pyrazol-4-yl)-N-ethyl-3-((3,3,3-trifluoropropyl)sulfite Acyl) propionamide (II)
[0087]
[0088] Under stirring at room temperature, to N-(3-chloro-1-(pyridin-3-yl)-1H-pyrazol-4-yl)-N-ethyl-3-((3,3,3-trifluoro A solution of propyl)thio)propionamide (I) (500 mg, 1.229 mmol) in hexafluoroisopropanol (5 mL) was added with 30% hydrogen peroxide (523 mg, 4.92 mmol). The solution was stirred for 15 minutes at room temperature. Quench it with saturated sodium sulfite solution and use CH 2 Cl 2 extraction. Silica gel chromatography (0%-10%MeOH / CH 2 Cl 2 ) Gives the title compound as a white semi-solid (495 mg, 95%): IR (film) 1660 cm -1 ; 1 H NMR(400MHz, CDCl 3 )δ8.96(d,J=2.4Hz,1H), 8.64(dd,J=4.7,1.4Hz,1H), 8.07–8.00(m,2H),7.46(ddd,J=8.3,4.8,0.7Hz ,1H),3.85-3.61(m,2H),3.23-3.08(m,1H),3.03-2.76(m,3H),2.74-2.52(m,4H),1.18(t,J=7.2Hz,3H ); ESIMS m / z 423([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com