Novel high-activity allinase and preparation method thereof
An alliinase, high-activity technology, applied in the field of bioengineering, can solve problems such as difficult to obtain satisfactory results, and achieve the effects of simplifying production links and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
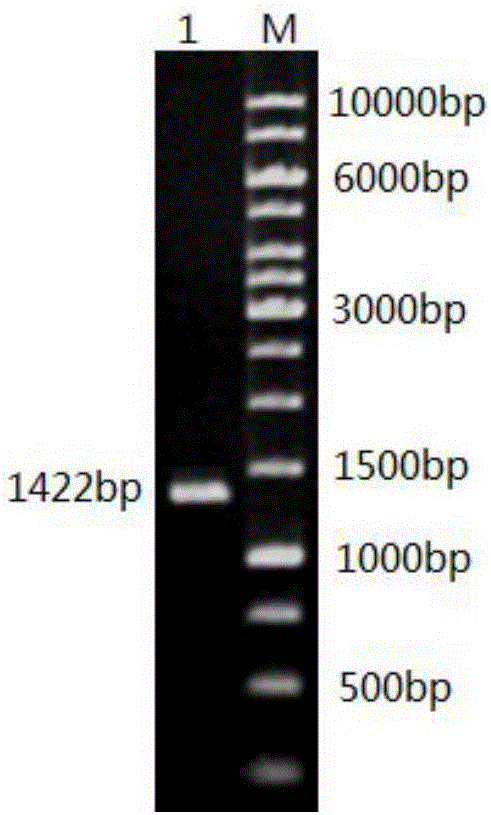
[0048] Obtaining the Mature Peptide Gene of Wild Type Alliinase
[0049] 1. Take fresh garlic bulbs and grind them in liquid nitrogen, extract total RNA according to the instructions of Trizol reagent and perform RT-PCR.
[0050] 2. Using the first strand of the synthesized cDNA as a template, design a pair of primers in the upstream and downstream of the ORF frame according to the alliinase sequence registered in Genbank sequence number S73324.1, and introduce restriction enzyme sites BamH I and Hind III respectively . Design the amplification primers of alliinase mature peptide gene of the present invention as follows:
[0051] Upstream primer P1 (SEQ ID NO.1): 5'-CGC GGATCC ATGATCTGCCTAGTGATTTTGACATG-3'
[0052] Downstream primer P2 (SEQ ID NO.2): 5'-CCC AAGCTT TTAAATGAAAGGACGACGGGAG-3'
[0053] The reaction conditions for its amplification are:
[0054]
[0055]
[0056] The amplification conditions were: pre-denaturation at 95°C for 5 min; denaturation at 95...
Embodiment 2
[0058] Acquisition of a novel high-activity alliinase gene.
[0059] 1. The recombinant plasmid was transformed into Escherichia coli DH5α, and the wild-type alliinase gene was successfully cloned into the pET28a vector after verification by BamH I and Hind III double enzyme digestion.
[0060] 2. Random mutation
[0061] (1) Perform random mutation based on error-prone PCR technology to construct a new type of high-activity alliinase, and design primers as follows:
[0062] Upstream primer P1 (SEQ ID NO.1): 5'-CGC GGATCC ATGATCTGCCTAGTGATTTTGACATG-3'
[0063] Downstream primer P2 (SEQ ID NO.2): 5'-CCC AAGCTT TTAAATGAAAGGACGACGGGAG-3'
[0064] In the error-prone PCR reaction system, P1 and P2 were used as upstream and downstream primers, and the wild-type alliinase mature peptide gene was used as a template to perform error-prone PCR.
[0065] The reaction conditions for its amplification are:
[0066]
[0067]
[0068] The amplification conditions were: pre-dena...
Embodiment 3
[0084] Construction of Novel High-Active Alliinase Recombinant Bacteria of Bacillus subtilis
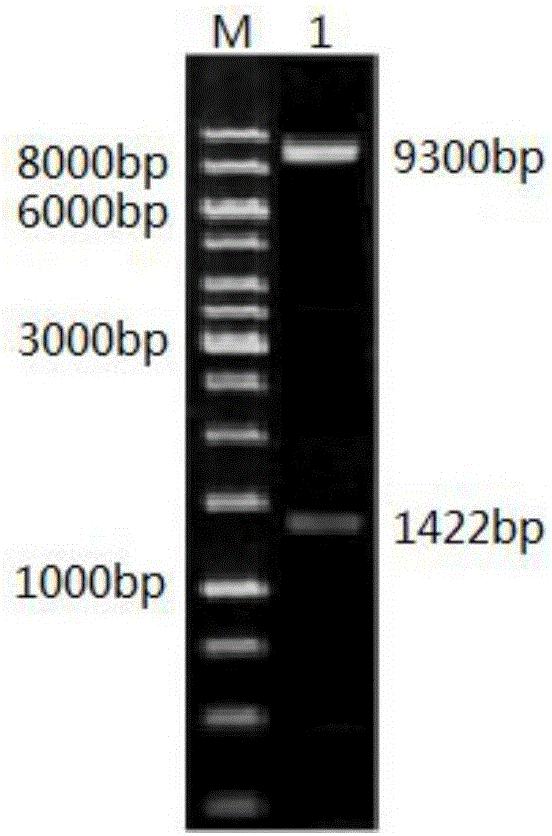
[0085] 1. Construction of expression vector pBSA43
[0086]pBSA43 is based on the Escherichia coli-Bacillus subtilis shuttle cloning vector pBE2 as the backbone, cloned into a strong Bacillus constitutive promoter P43, and the fructan sucrase signal sequence sacB that can directly secrete the recombinant protein into the medium . it comes with amp r Gene that can use ampicillin resistance as a selectable marker in E. coli; also has Km r Gene, kanamycin resistance can be used as a selection marker in Bacillus subtilis and Bacillus licheniformis.
[0087] 2. Construction of a novel high-activity alliinase expression vector pBSA43-alliinasem
[0088] The novel high-activity alliinase gene obtained by error-prone PCR construction was double-digested with BamHI and HindIII and then ligated with the same double-digested Bacillus subtilis expression vector pBSA43 to construct the recomb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com