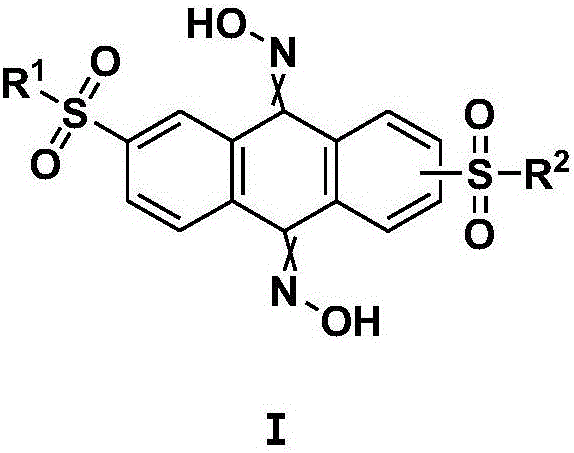
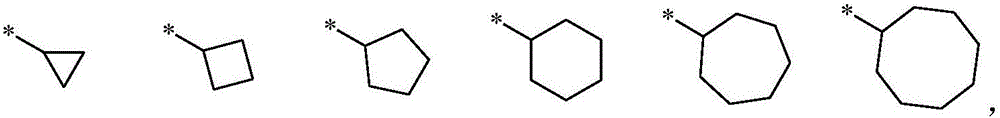
Bisulfonyl anthracenedione dioxime derivative serving as P2X<3> and P2X<2/3> receptor antagonists
A sulfonyl and receptor technology, applied in the field of medicinal chemistry, can solve the problems of long-distance and long-term signal transmission and its mechanism is not very clear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
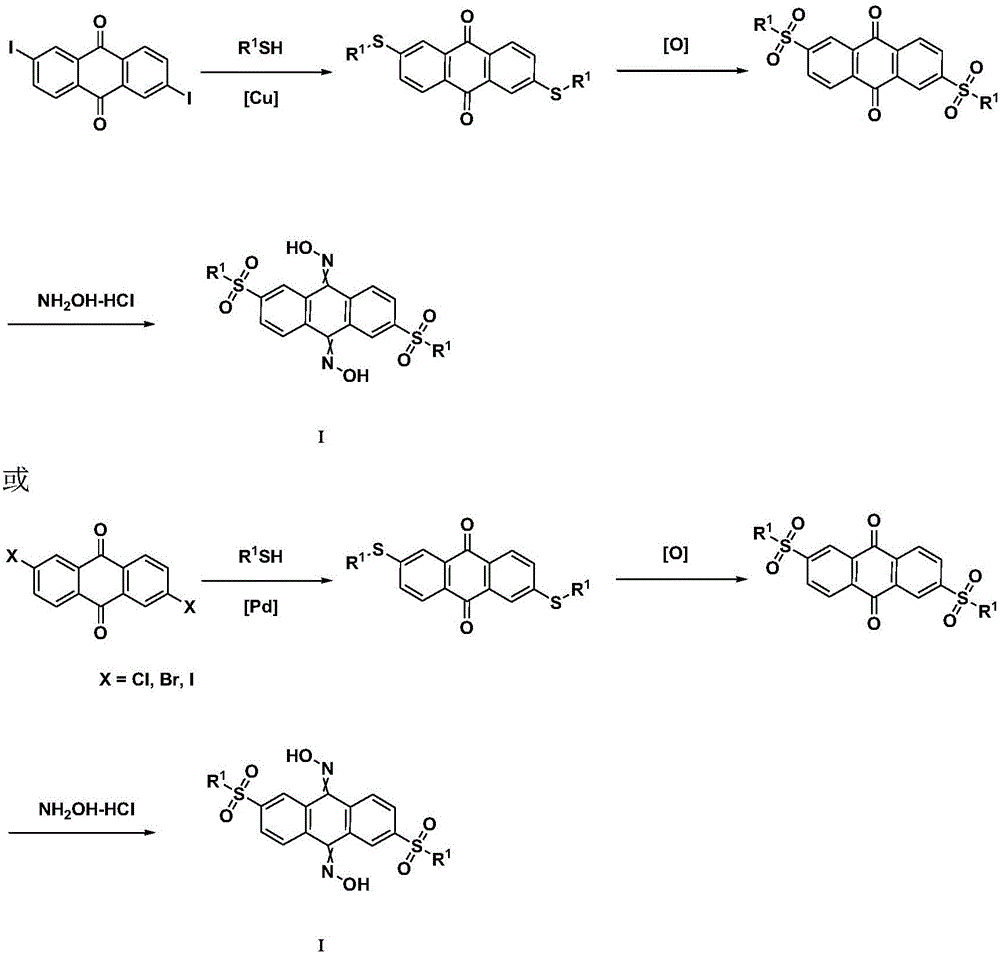
[0044] Step 1: Preparation of 2,7-bis(cyclohexylmercapto)-anthracene-9,10-dione
[0045]
[0046] Under nitrogen protection, 2,7-diiodoanthracene-9,10-dione (11.5g, 25mmol), cyclohexyl mercaptan (3.48g, 30mmol), cesium carbonate (24.4g, 75mmol), palladium acetate ( 113mg, 0.5mmol), (±) BINAP (155mg, 0.25mmol) was added to 120mL DMF, heated to 120°C, after the reaction was detected by TLC, the temperature was lowered. Add 200mL of water to the reaction solution, extract with ethyl acetate (200mL×3), collect the organic phase and dry it with anhydrous sodium sulfate, concentrate under reduced pressure, the crude product is obtained by silica gel column chromatography (PE / EA=80 / 20) to obtain 2 , 9.99 g of 7-bis(cyclohexylmercapto)-anthracene-9,10-dione (yield: 91.6%). LC / MS(M+1) + = 436.2.
[0047] Step 2: Preparation of 2,7-bis(cyclohexylsulfonyl)-anthracene-9,10-dione
[0048]
[0049] 2,7-bis(cyclohexylmercapto)-anthracene-9,10-dione (4 g, 9 mmol) was dissolved in 10...
Embodiment 2
[0054] Step 1: Preparation of 2,6-bis(cyclohexylmercapto)-anthracene-9,10-dione
[0055]
[0056] Under nitrogen protection, 2,6-dichloroanthracene-9,10-dione (6.9g, 25mmol), cyclohexyl mercaptan (3.48g, 30mmol), cesium carbonate (24.4g, 75mmol), palladium acetate ( 113mg, 0.5mmol), (±) BINAP (155mg, 0.25mmol) was added to 120mL DMF, heated to 120°C, after the reaction was detected by TLC, the temperature was lowered. Add 300mL of water to the reaction solution, extract with ethyl acetate (200mL×3), collect the organic phase, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and use silica gel column chromatography (PE / EA=80 / 20) to obtain 2 , 10.3 g of 6-bis(cyclohexylmercapto)-anthracene-9,10-dione (yield: 94.2%). LC / MS(M+1) + = 436.2.
[0057] The second step: the preparation of 2,6-di(cyclohexylsulfonyl)-anthracene-9,10-dione
[0058]
[0059] 2,6-bis(cyclohexylmercapto)-anthracene-9,10-dione (8 g, 18 mmol) was dissolved in 100 mL of chlorofor...
Embodiment 3
[0064] 2,7-bis(cyclopentylsulfonyl)-anthracene-9,10-dioxime 1 HNMR and LC / MS
[0065]
[0066] 1 H-NMR (400MHz, DMSO-d 6 ): δ9.03(d, J=1.6Hz, 1H), 9.01(d, J=1.6Hz, 1H), 8.75(d, J=8.0Hz, 1H), 8.68(d, J=8.4Hz, 1H ),8.63-8.56(m,2H),3.68-3.60(m,2H),3.19-2.99(m,8H),2.37-2.27(m,8H);LC / MS(M+1) + = 503.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com