Use of lentinan in preparation of drug for preventing diabetes type 1
A technology of type 1 diabetes and lentinan, applied in the field of medicine, can solve the problem of not knowing that lentinan is equally effective for type 1 diabetes, and achieve the effects of improving serum insulin level, improving survival rate and relieving insulitis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
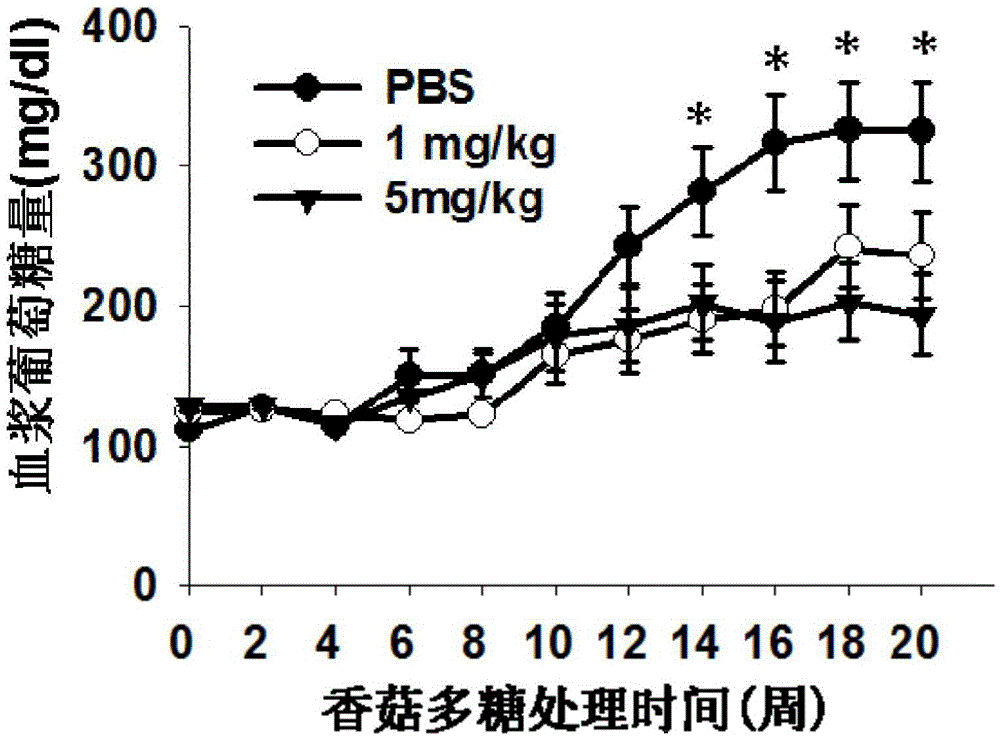
[0033] Example 1 Effect of lentinan on the onset of type 1 diabetes in NOD mice.
[0034] Experimental animals: 4-5-week-old female NOD mice and feed, provided by Changzhou Cavens Experimental Animal Co., Ltd., license number: SCXK (Su) 2011-0003. Animals were housed in random cages, with free access to food and water.
[0035] Experimental drugs: Lentinan was purchased from Jiangsu Yongjian Pharmaceutical Technology Co., Ltd., and the negative control was PBS.
[0036] Experimental steps:
[0037] 1) 4-week-old female NOD mice entered the Experimental Animal Center of Nanjing Medical University and were reared in a clean environment with a temperature of (21±2)°C and a humidity of (35±2)%, 12h:12h day and night without lighting, free to eat and drink, Drinking water was distilled water prepared by the Experimental Animal Center.
[0038] 2) After one week of adaptive feeding, they were randomly divided into 3 groups, 15 in each group, and were injected intraperitoneally wi...
Embodiment 2
[0052] Example 2 Effect of lentinan on the levels of pro-inflammatory and anti-inflammatory cytokines in serum of NOD mice.
[0053] According to the kit instructions, the ELISA method was used to detect the levels of pro-inflammatory and anti-inflammatory cytokines in the mouse serum of Example 1, and the differences between the PBS control group and the lentinan experimental group were compared. The specific steps are:
[0054] 1) Add serum samples and standards of different concentrations (100 μl / well) to the corresponding wells, seal the reaction wells with sealing tape, and incubate at 37°C for 90 minutes (except for blank control wells);
[0055] 2) Shake off the liquid in the wells, add 350 μl of washing solution to each well, let it stand for 30 seconds, shake off the liquid, pat dry on absorbent paper, and repeat this step 4 times;
[0056] 3) Add biotinylated antibody working solution (100 μl / well), seal the reaction well with sealing tape, and incubate at 37°C for ...
Embodiment 3
[0063] Example 3 Effect of Lentinan on CD25 in spleen and pancreatic lymph nodes of NOD mice + Foxp3 + Treg, CD3 + CD8 + Effect of CTLs.
[0064] Analysis of CD25 in mouse spleen and pancreatic lymph nodes in Example 1 by flow cytometry + Foxp3 + Treg and CD3 + CD8 + For the number of CTL cells, compare the differences between PBS and lentinan experimental groups. Specific steps are as follows:
[0065] 1) Put 3 groups of cleaned fresh mouse spleen or pancreatic lymph nodes in a plate filled with RPMI-1640 cell culture medium, grind the spleen or pancreatic lymph nodes with a glass homogenizer, filter and pour into a graduated centrifuge tube, Add culture medium to 6ml;
[0066] 2) 1500rpm / min at room temperature, centrifuge for 5min;
[0067] 3) Discard the supernatant, add Tris-NH to the lower layer of cells 4 Cl (pH7.0), shake for 2 minutes to lyse and destroy red blood cells;
[0068] 4) Add PBS (pH 7.0) containing 0.5% BSA to 6ml, centrifuge at 1500rpm / min at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com