Reprogramming of pluripotent stem cells for improved control of their differentiation pathways
A pluripotent stem cell and reprogramming technology, which is applied in the field of pluripotent stem cell reprogramming to improve the control of pluripotent stem cell differentiation pathways, and can solve the problems of being unable to transport PSCs over long distances and not transporting PSCs for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
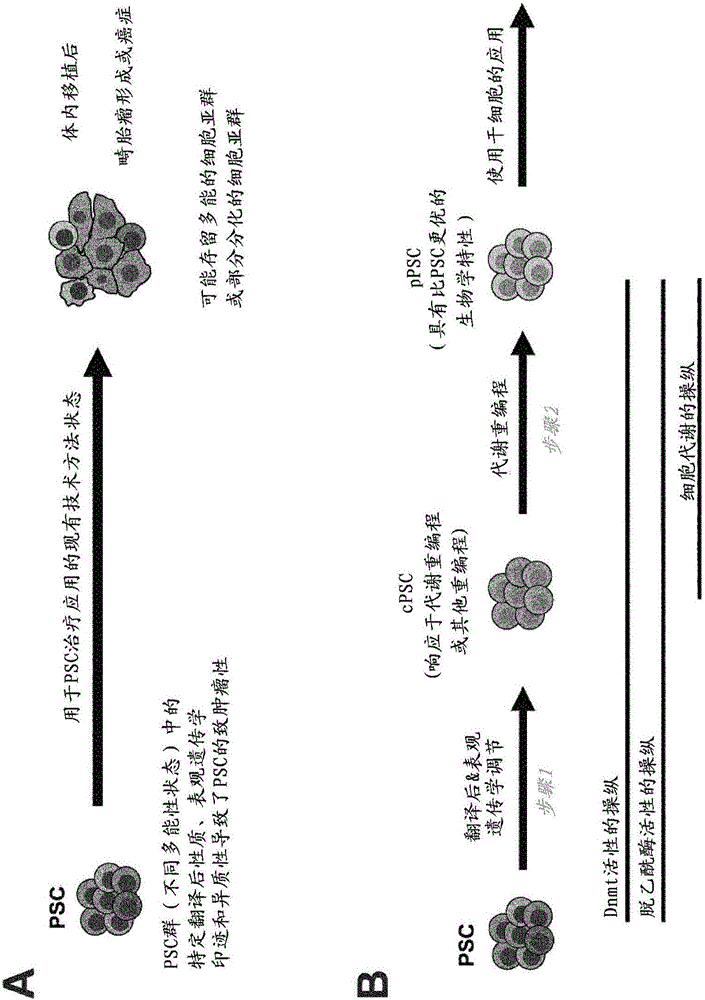
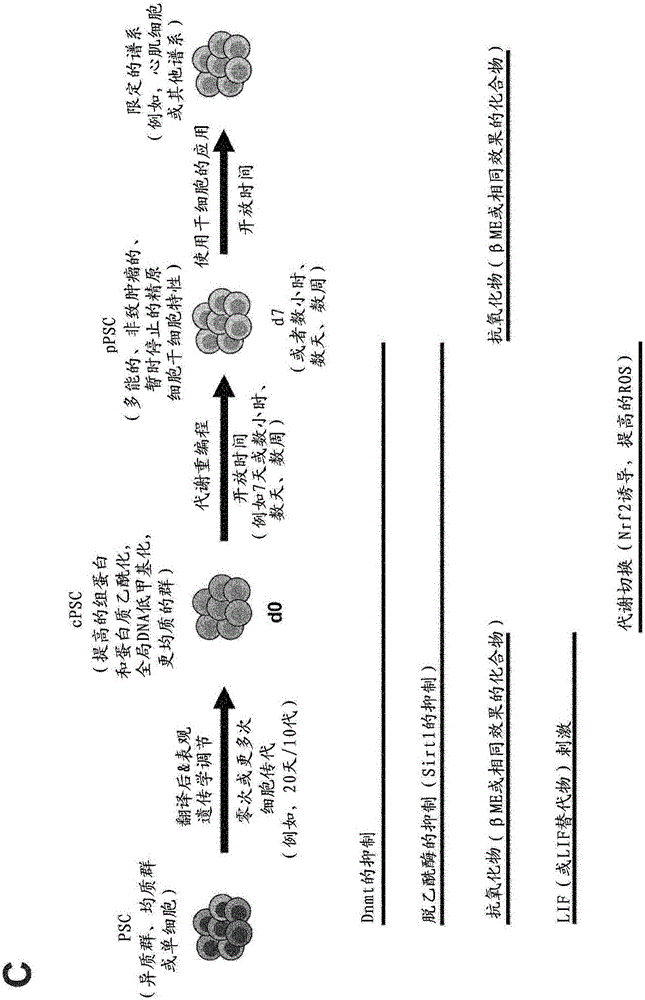
[0150] The present invention relates to reprogramming pluripotent stem cells (PSCs) comprising at least two steps: converting the PSCs to epigenetically regulated and optionally post-translationally regulated PSCs (cPSCs) (step a), and converting The conditioned cPSCs are transformed into metabolically reprogrammed PSCs (pPSCs) (step b).
[0151] Therefore, in a first aspect, the present invention relates to a method for reprogramming pluripotent stem cells (PSCs), comprising the steps of:
[0152] (a) epigenetic regulation of PSCs, and
[0153] (b) Metabolic reprogramming of PSCs.
[0154] Preferably, in step (a) the PSCs are conditioned to cPSCs (conditioned pluripotent stem cells), preferably preparing the cells for subsequent processing, ie making them responsive to the metabolic reprogramming of step (b). Preferably, in step (b), cPSCs are metabolically reprogrammed into pPSCs (reprogrammed pluripotent stem cells).
[0155] The method of the first aspect of the inventi...
Embodiment 1
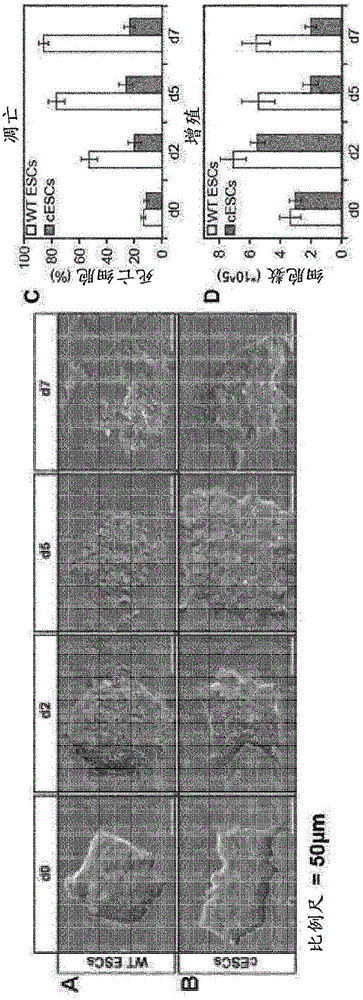
[0344] During 7 days (d0 to d7), mouse ESCs (mESCs) were conditioned, converted to cPSCs (cESCs) and subsequently reprogrammed into pPSCs using the methods described in the present invention.
[0345] General Cell Culture
[0346]As a model of PSC, mESC was purchased from Millipore (Millipore, Zug, Switzerland, MilliTrace TM NanogGFP reporter cell line, SCR089). These mESCs have a puromycin resistance gene and a NanogGFP reporter (GFP coding sequence fused to the Nanog promoter) and can be used as an internal control for pluripotency. All cells were cultured in T25, T75 tissue culture flasks (TPP, Trasadingen, Switzerland) or 8-well μ-Slides (ibidi, Munich, Germany, μ-Slides 8-well ibiTreat, tissue culture treatment, 80826) in the atmosphere containing 5% CO 2 A humidified atmosphere of 37°C CO 2 In the incubator, the tissue culture flasks and 8-well μ-Slides were coated with EmbryoMax gelatin solution (0.1%) (Millipore, ES-006-B) for 15 minutes at room temperature before s...
Embodiment 2
[0362] Example 2 - Protein levels of pluripotency markers during and after metabolic reprogramming
[0363] To analyze the pluripotency of cESCs during metabolic reprogramming, by immunofluorescence ( Figure 4 ) and Western blot analysis ( Figure 5 ) to analyze protein levels of pluripotent markers.
[0364] Western blot analysis was performed as described in Example 1. The primary antibody and its dilution are 1:1 for Nanog (rabbit, Millipore, ab9220), Sox2 (mouse, Millipore, mab4343), phosphorylated Stat3 (rabbit, Cell Signaling Technology, 9131), total Stat3 (mouse, Cell Signaling Technology, 9139) 1000; 1:500 for Oct4 (rabbit, Millipore, ab3209).
[0365] Immunofluorescence
[0366]Cells were fixed with formaldehyde (2.5% w / v) in PBS for 20 min, permeabilized with 0.3% Triton-X100 in PBS for 10 min, and incubated with 2% albumin fraction V (Applichem, Darmstadt, Germany). Block in PBS for 1 hr at room temperature. Cells were then treated with anti-Nanog (rabbit, Mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com