Compounds for treatment of cancer and epigenetics
A compound and alkyl technology, applied in the field of quinoline and 5,6,7,8-tetrahydroacridine derivatives, can solve the problems of undisclosed anti-proliferation cell activity and achieve high efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0361] list of abbreviations used
[0362]
[0363] Bioassay Materials and Methods
[0364] SMYD3 biochemical assay
[0365] The SMYD3 enzymatic assay was developed using Promega's methyltransferase-Glo™ reagent. In the assay, SMYD3 catalyzes the methylation of MAP3K2 peptide substrates by transferring a methyl group from SAM to MAP3K2 peptide and further converts SAM to SAH. SMYD3 methyltransferase activity was measured based on the amount of SAH produced from the reaction by using a coupled enzyme that converts SAH to ATP. The MTase-Glo detection solution then catalyzes the formation of light from ATP.
[0366] for IC 50 For determination, compounds were incubated with 0.4 μM SMYD3 enzyme for 30 min in low volume 384-well plates. SAM and MAP3K2 peptides were added at final concentrations of 1.0 μM and 10 μM and further incubated for 30 min at room temperature before adding MTase Glo and detection reagents. Reaction signals were detected using a microplate reader (...
Embodiment 2
[0380] SMYD3 biochemical assay
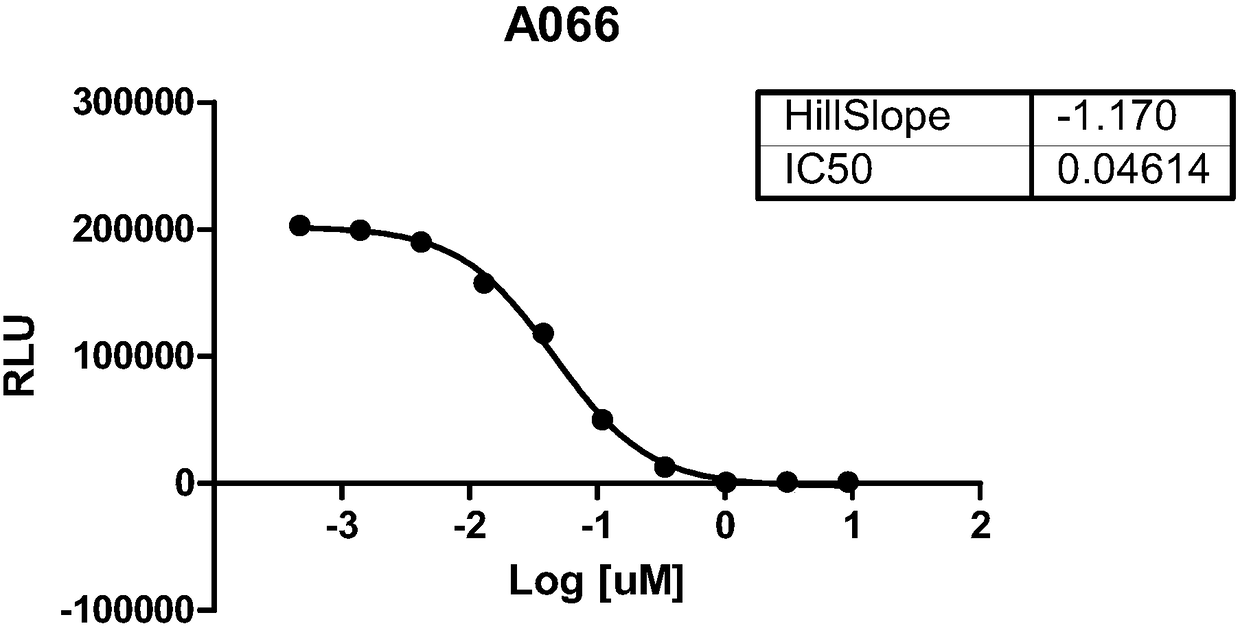
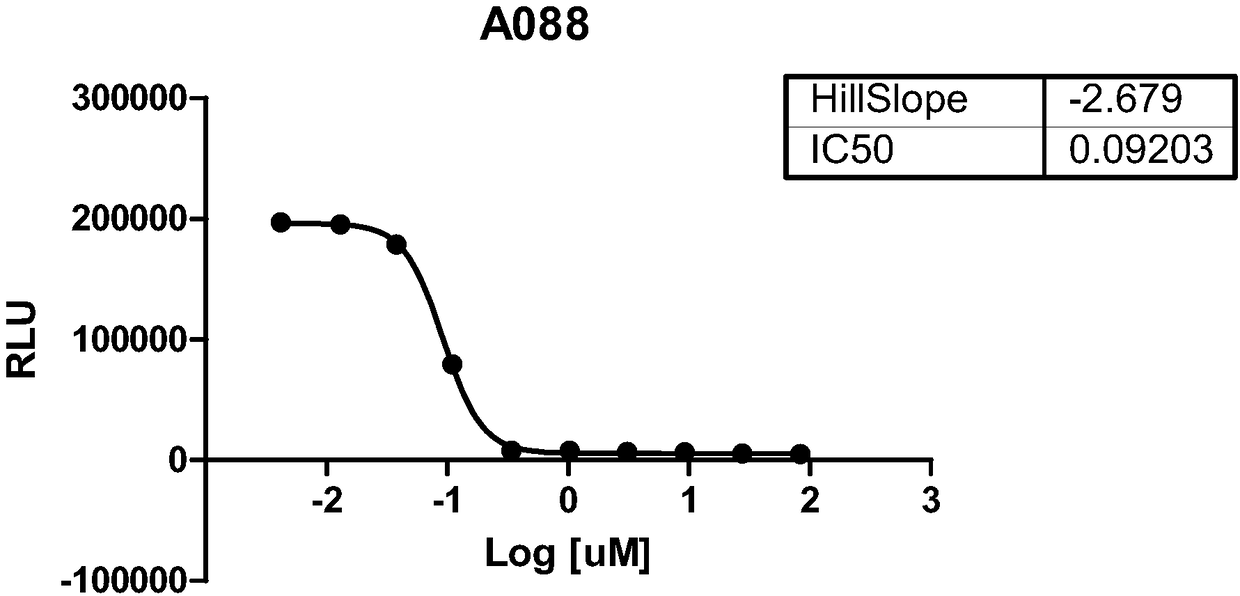
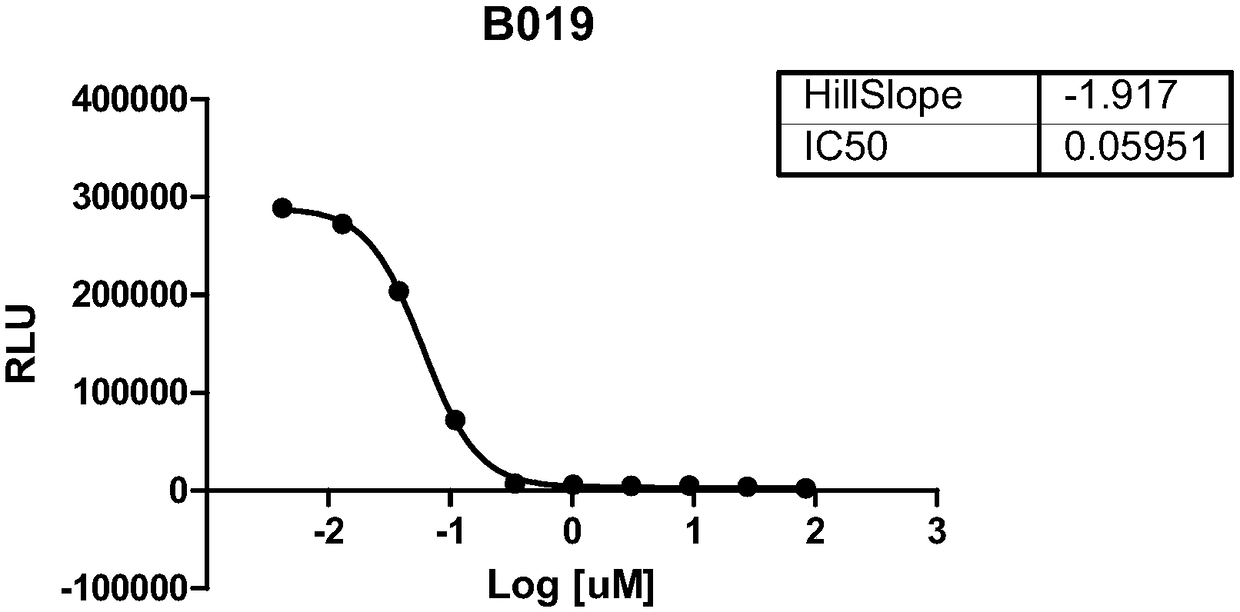
[0381] The ability of compounds to inhibit the catalytic function of SMYD3 was tested using the MTase assay by using MAP3K2 as the peptide substrate. Said compounds were found to inhibit the methyltransferase activity of SMYD3. The effect of the compounds on the methyltransferase activity of SMYD3 using the MAP3K2 peptide as substrate can be found in Figure 1 (Compounds A066; A074; B019; A088). The data have been summarized in Table 1.
[0382] Table 1. Biochemical ICs of SMYD3 Inhibitors 50 summary.
[0383] compound
Embodiment 3
[0385] SMYD3 compounds inhibit the proliferation of different cancer cells
[0386] The antiproliferative effect of SMYD3 inhibition was explored in different cancer cell lines. Dose-response curves are shown in FIG. 2 . All cell lines with GI ranging from 11 to 50 μM 50 Values are in response to SMYD3 inhibitors. A subset of the data is summarized in Table 2.
[0387] Table 2. Summary of antiproliferative activity of SMYD3 inhibitors in different cancer cell lines (compounds A066; A074; B019; A088).
[0388]
[0389] *ND: not determined
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com