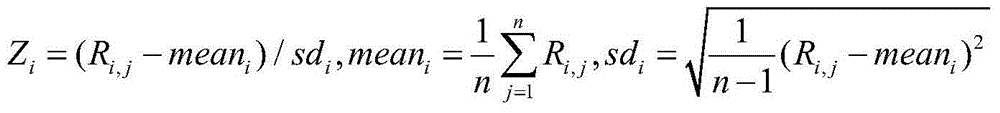Standard product for polyploid chromosome detection and preparation method thereof
A technology for standards and chromosomes, which is applied in biochemical equipment and methods, and the determination/inspection of microorganisms.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
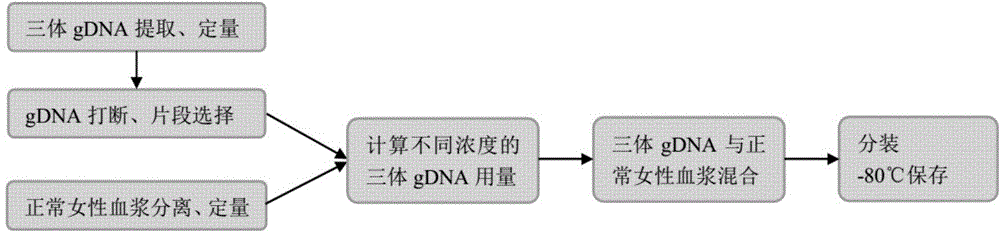
[0082] Preparation method of standard product
[0083] From a technical point of view, the present invention usually has the following four methods to prepare simulated samples:
[0084] 1) Genomic DNA extracted from known trisomy 21 patients or trisomy 21 cell lines is mixed with normal female genomic DNA in proportion, interrupted to the size distribution range of plasma DNA fragments, and set aside;
[0085] 2) Genomic DNA extracted from known trisomy 21 patients or trisomy 21 cell lines is used to break into the size distribution range of plasma DNA fragments, and mixed with the disrupted products of normal female genomic DNA in proportion;
[0086] 3) Genomic DNA extracted from patients with trisomy 21, 18, and 13 or cell lines with trisomy 21, 18, and 13 was used to break into the size distribution range of plasma DNA fragments, and plasma DNA separated from normal female peripheral blood mix in proportion;
[0087] 4) Plasma DNA isolated from peripheral blood of patie...
Embodiment 1
[0177] The influence of embodiment 1 different size trisomy DNA fragments on standard
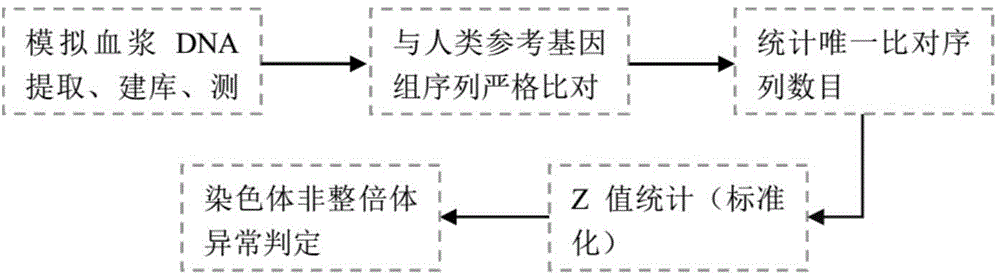
[0178] This embodiment simulates the influence of three T21 gDNA fragment sizes (100-150bp, 150-200bp, 200-250bp) on the prepared plasma sample with abnormal fetal chromosome number when the fetal concentration is 10%.
[0179] The results are shown in Table 1. It can be seen from the results that the abnormal number of fetal chromosomes can be detected in the plasma samples prepared by DNA with three fragment sizes. Compared with the data, when the fragment size is selected as 150-200bp, the Z values of the three platforms can all be tested. Stable above 3.50 and 150-200bp is the real situation of the fragment distribution size of free DNA in plasma. Based on the above considerations, it is preferred to choose trisomy gDNA with a fragment size of 150-200bp to interrupt and purify the DNA and mix it with normal female plasma to prepare the standard Taste.
[0180] Table 1
[0181]
...
Embodiment 2
[0183] The impact of embodiment 2 different polyploidy chromosome concentration on standard substance detection accuracy
[0184] According to the existing reports that triploids account for the percentage (3-20%) of the concentration of free DNA in the mother's plasma, using the general method, this embodiment simulates the concentration of the fetus as 1%, 2%, 3%, 3.5%, 4%, 5%. %, 7%, 10%, 12% and other 9 concentration gradients of T21 plasma samples to select the most suitable gradient as a standard.
[0185] The results can be seen in Table 2. Combined with the results of sequencing and information analysis, it can be seen that at about 3.5%, the Z value of chromosome 21 has a critical value, so about 3.5% can be selected as the minimum detection concentration value; and when the concentration is above 4%. , the Z value can show a relatively stable detection rate; when the fetal concentration is 10%, the detection rates of the three platforms are all 100%, and the simulate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com