Vilazodone hydrochloride crystal form and preparation method thereof
A vilazodone hydrochloride crystal form and a technology for vilazodone hydrochloride are applied in the field of new vilazodone hydrochloride crystal form A and its preparation, and can solve the problems of low rate of defective products in tablets and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: Preparation of vilazodone hydrochloride crystal form A
[0069] ①Under room temperature, dissolve vilazodone powder (12g, 27.2mmol) in THF (tetrahydrofuran) (240mL), slowly add water (480mL) into the solution under stirring, after the addition is complete, stir at room temperature for 30min;
[0070] ② Add 2M hydrochloric acid aqueous solution (24.5mL, 49mmol) to the solution system, cool down to 10°C, stir for 2-3h, and crystallize;
[0071] ③ suction filtration, filter cake water (48mL) rinse;
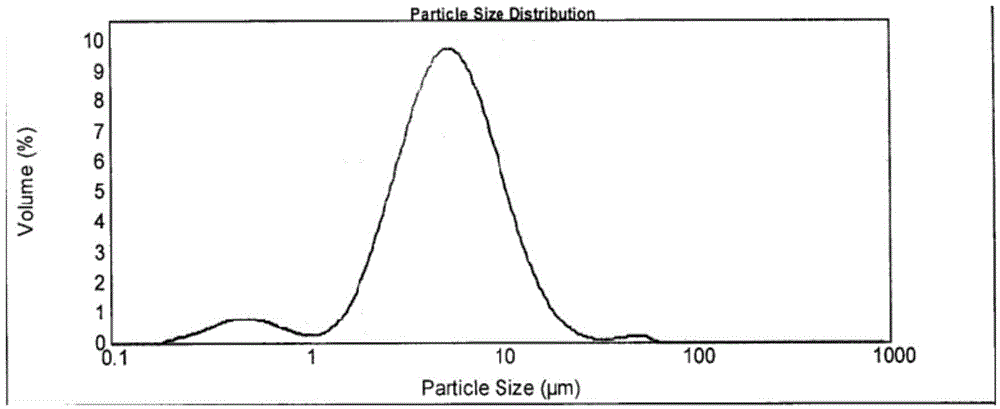
[0072] ④ The obtained solid is then vacuum-dried at 50°C-60°C (vacuum degree ≥ 0.09MPa), and dried for 2h to obtain vilazodone hydrochloride crystal form A of the present invention (11.5g, yield 88.5%, purity (HPLC detection) is 99.78%).
[0073] Measure the vilazodone hydrochloride crystal form A obtained in step 4., as follows:
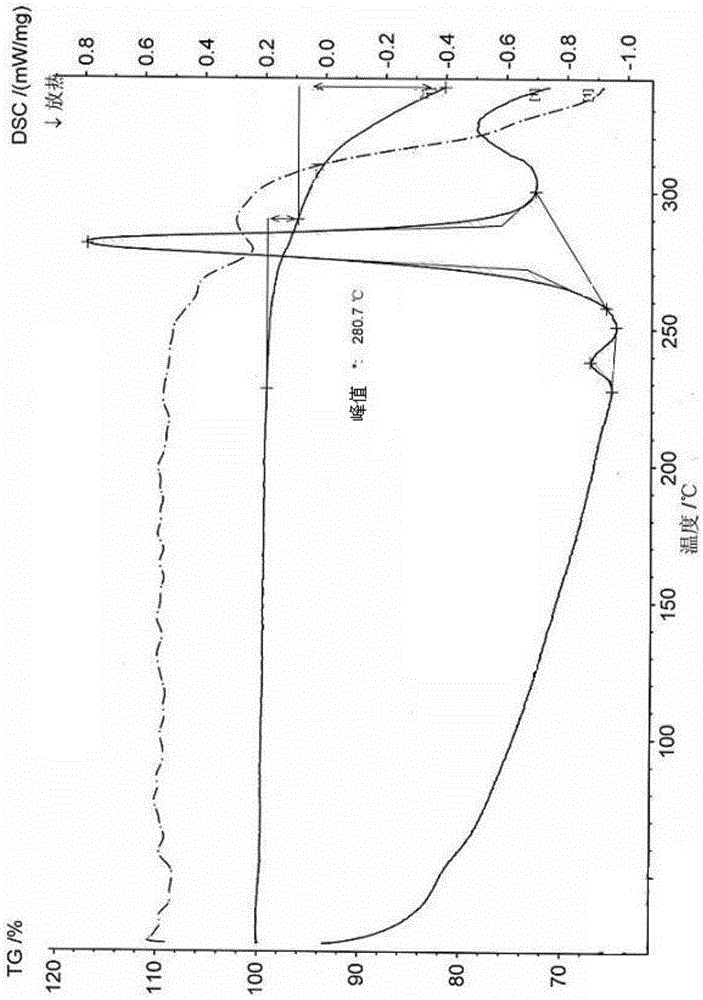
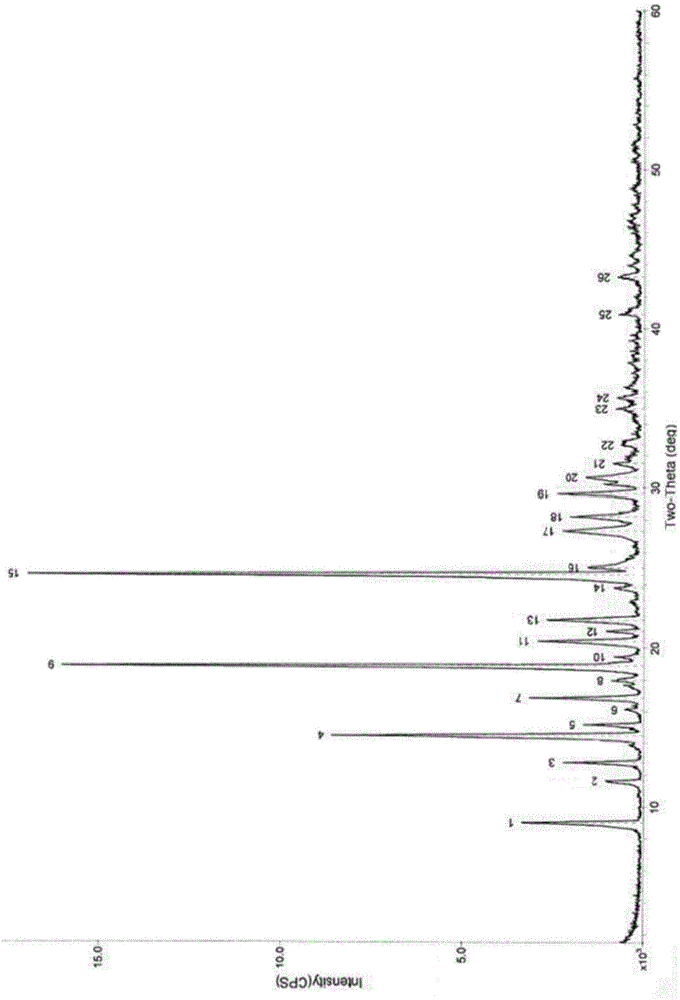
[0074] (1) Powder X-ray Diffraction Measurement Results
[0075] The powder X-ray diffraction pattern of table 1 vilazodone hydro...
Embodiment 2-4
[0084] Example 2-4: Preparation of vilazodone hydrochloride crystal form A
[0085] With reference to the preparation process of Example 1, the consumption of purified water in step 2. is changed to 528mL, 600mL, 720mL respectively, that is, the water (H 2 O) and the volume ratio of tetrahydrofuran in step ① (see Table 3 below for details), respectively, to obtain vilazodone hydrochloride crystal form A of the present invention.
[0086] Table 3 water and tetrahydrofuran volume ratio investigation result
[0087]
Embodiment 5~6
[0093] Embodiments 5-6: Preparation of vilazodone hydrochloride crystal form A
[0094] With reference to the preparation process of Example 1, only the volume of the 2M hydrochloric acid added in step ② was changed to 20.4mL (40.8mmol) and 27.2mL (54.4mmol) respectively, that is, the volume ratio of hydrochloric acid in step ② to tetrahydrofuran in step ① was changed The obtained vilazodone hydrochloride yield and purity (HPLC detection) of the present invention are specifically shown in Table 5 below.
[0095] Table 5 hydrochloric acid addition investigation result
[0096]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com