Method for separating Fe2O3 in pulverized coal ash of circulating fluidized bed
A circulating fluidized bed, fly ash technology, applied in the direction of iron oxide, iron oxide/iron hydroxide, etc., can solve the problems of poor implementation effect of iron oxide, low recovery rate of iron oxide, and limitation of process conditions, etc., to achieve broadening and comprehensive The range of utilization, large processing capacity and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
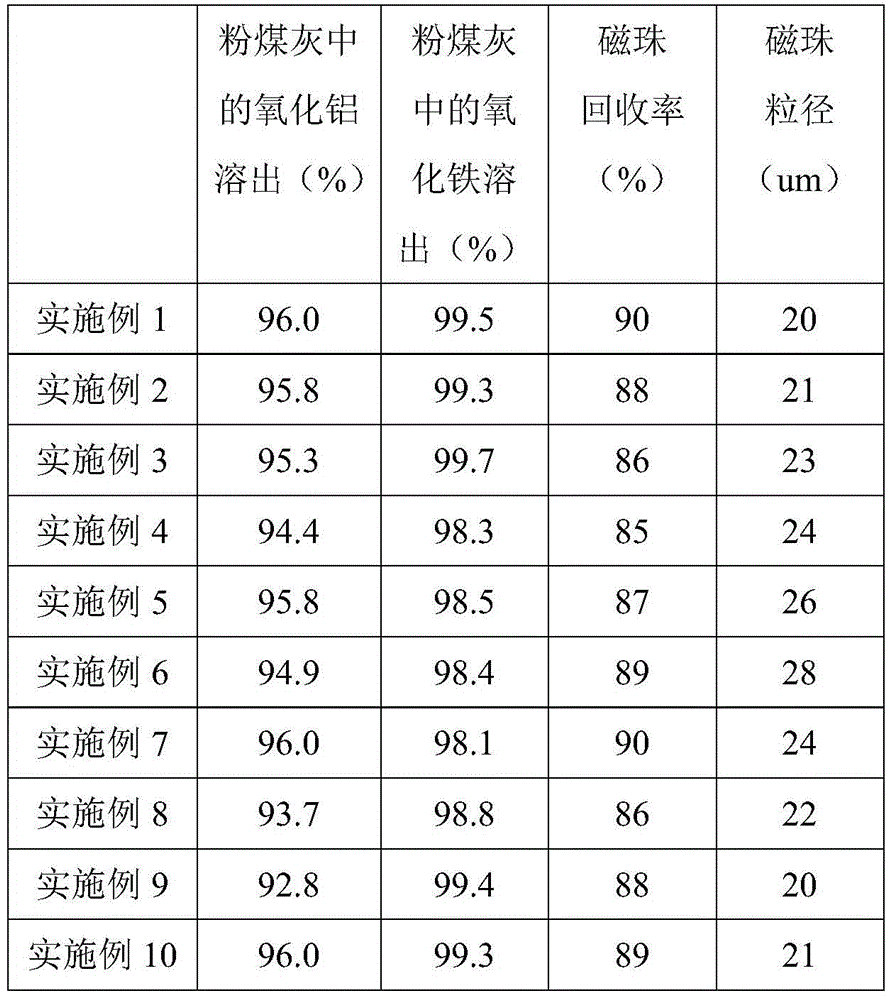
[0033] Example 1, the fly ash was subjected to 4 times of magnetic separation, then mixed with 5% sulfuric acid at a solid-to-liquid ratio of 1:1, and reacted at 80°C for 4 hours; after the reaction was completed and cooled to room temperature, the filter cake was obtained by suction filtration, And wash with water with a solid-to-liquid ratio of fly ash to water of 1:1 to obtain a solution of ferric sulfate and aluminum sulfate; prepare acetone at a volume ratio of 3:1 to the solution of ferric sulfate and aluminum sulfate, add acetone, and stir for 5 minutes , suction filtration to obtain aluminum sulfate filter cake, and reclaim the filtrate; mix aluminum sulfate filter cake with 70% acetone aqueous solution at a ratio of 1:3, stir for 15 minutes, and suction filter to obtain aluminum sulfate filter cake, and recycle Filtrate: Repeat the washing process with acetone aqueous solution for 6 times, and recover the filtrate; mix hydrogen peroxide and recovered filtrate at a volu...
Embodiment 2
[0034] Example 2, after 3 times of magnetic separation, the fly ash was mixed with 10% sulfuric acid at a solid-to-liquid ratio of 1:3, and reacted at 90°C for 6 hours; after the reaction was completed and cooled to room temperature, the filter cake was obtained by suction filtration, And wash with water with a solid-to-liquid ratio of fly ash to water of 2:1 to obtain a solution of iron sulfate and aluminum sulfate; prepare acetone at a volume ratio of acetone to iron sulfate and aluminum sulfate solution of 2:1, add acetone, and stir for 3 minutes , suction filtration to obtain aluminum sulfate filter cake, and reclaim the filtrate; mix aluminum sulfate filter cake with 60% acetone aqueous solution at a ratio of 1:5, stir for 30 minutes, and suction filter to obtain aluminum sulfate filter cake, and recycle Filtrate: repeat this acetone aqueous solution washing process 4 times, and recover the filtrate; mix the hydrogen peroxide and the recovered filtrate at a volume ratio of...
Embodiment 3
[0035]Example 3, after 3 times of magnetic separation, the fly ash was mixed with 1% sulfuric acid at a solid-to-liquid ratio of 1:3, and reacted at 60°C for 6 hours; after the reaction was completed and cooled to room temperature, the filter cake was obtained by suction filtration, And wash with water with a solid-to-liquid ratio of fly ash to water of 2:1 to obtain a solution of iron sulfate and aluminum sulfate; prepare acetone according to the volume ratio of acetone to iron sulfate and aluminum sulfate solution of 1:1, add acetone, and stir for 5 minutes , filtered with suction to obtain an aluminum sulfate filter cake, and reclaim the filtrate; mix the aluminum sulfate filter cake with 50% acetone aqueous solution at a ratio of 1:2, stir for 10 minutes, and then suction filter to obtain an aluminum sulfate filter cake, and recycle Filtrate: repeat the washing process with acetone aqueous solution 6 times, and recover the filtrate; mix hydrogen peroxide and recovered filtr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com