Denitration catalyst carried by CeO2 nanotube and preparation method of denitration catalyst
A denitrification catalyst and nanotube technology, applied in the field of selective catalytic reduction denitrification catalyst and its preparation, can solve the problems of limited anti-poisoning performance, achieve good water resistance and anti-sulfur performance, prolong service life, and low sulfur dioxide oxidation rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
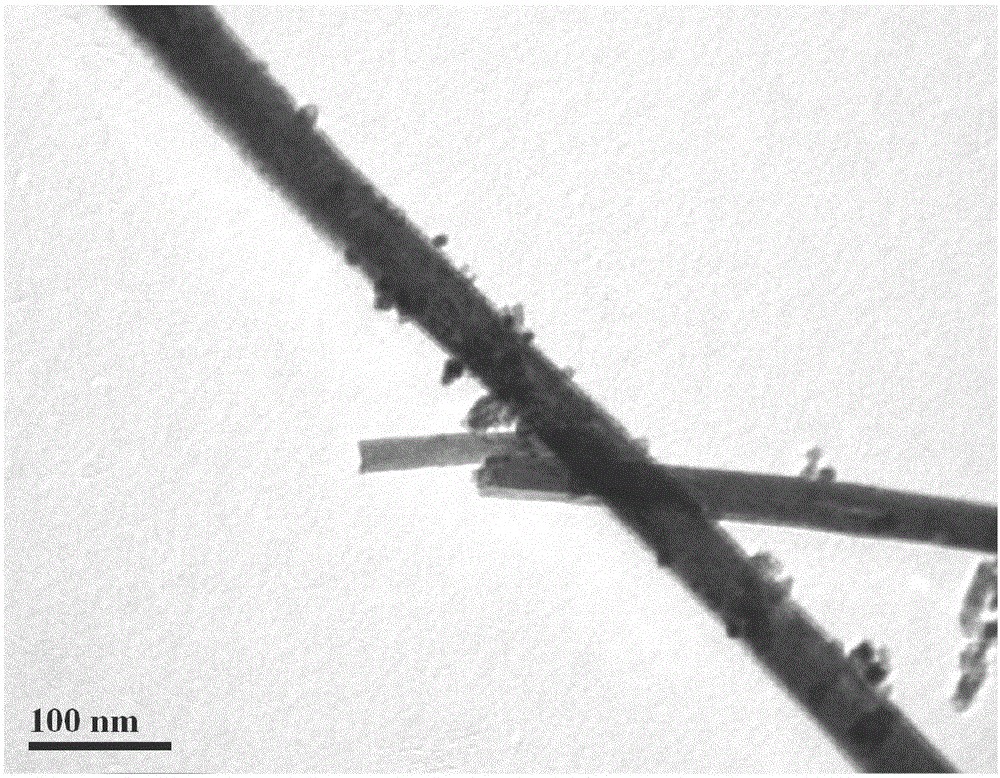
[0047] Preparation of ceria-titanium nanotubes: The molar ratio of raw materials is CeCl 3 ·7H 2 O: water: sodium hydroxide = 1:70:30. Sodium hydroxide was dissolved in deionized water to prepare a strong alkali solution, and at the same time, high-purity He gas was used to purify it for 1 hour to remove dissolved oxygen to ensure an oxygen-free solution environment, and then CeCl 3 ·7H 2 O was added to the strong alkali solution, sealed and stirred for 2 hours, then poured into a polytetrafluoro-lined hydrothermal kettle at 120°C for 24 hours, and the filling degree of the hydrothermal kettle was 70%. Wash the sample with ionized water until the pH value is 6.5. The deionized water used for washing should also ensure no dissolved oxygen, and then dry the sample at 60°C. The surface morphology of ceria-titanium nanotubes is shown in figure 1 .
[0048] Preparation of the catalyst: Dissolve 2 grams of sodium polyacrylate in 80 milliliters of deionized water, then add 2 gra...
Embodiment 2
[0052] Preparation of cerium oxide nanotubes: The molar ratio of raw materials is CeCl 3 ·7H 2 O: water: potassium hydroxide = 1:150:50. Dissolve potassium hydroxide in water to prepare a strong alkali solution, and use high-purity He gas to purify it for 2 hours to remove dissolved oxygen to ensure an oxygen-free solution environment, and then add CeCl 3 ·7H 2 O was added to the strong alkali solution, sealed and stirred for 6 hours, then poured into a polytetrafluoro-lined hydrothermal kettle at 150°C for 48 hours, and the filling degree of the hydrothermal kettle was 75%. Wash the sample with ionized water until the pH value is 6.5. The deionized water used for washing should also ensure no dissolved oxygen, and then dry the sample at 60°C.
[0053] Catalyst preparation: Dissolve 2 grams of polyacrylamide in 80 milliliters of deionized water, then add 4 grams of cerium oxide nanotubes into it for ultrasonic (40KHz) dispersion for 2 hours, then add 1 gram of copper sulfat...
Embodiment 3
[0057] Preparation of cerium oxide nanotubes: The molar ratio of raw materials is Ce(NO 3 ) 3 ·6H 2 O: water: sodium hydroxide = 1:100:40. Dissolve sodium hydroxide in water to prepare a strong alkali solution, purify it with He gas for 1 hour to remove the dissolved oxygen in it to ensure an oxygen-free solution environment, and then add Ce(NO 3 ) 3 ·6H 2 O was added to the strong alkali solution, sealed and stirred for 2 hours, then poured into a polytetrafluoro-lined hydrothermal kettle at 150°C for 36 hours, and the filling degree of the hydrothermal kettle was 70%. Wash the sample with ionized water until the pH value is 7. The deionized water used for washing should also ensure no dissolved oxygen, and then dry the sample at 80°C.
[0058] Preparation of the catalyst: Dissolve 2 grams of sodium polystyrene sulfonate in 80 ml of deionized water, then add 2 grams of cerium oxide nanotubes and 2 grams of ammonium tungstate to it for ultrasonic (40KHz) dispersion for 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com