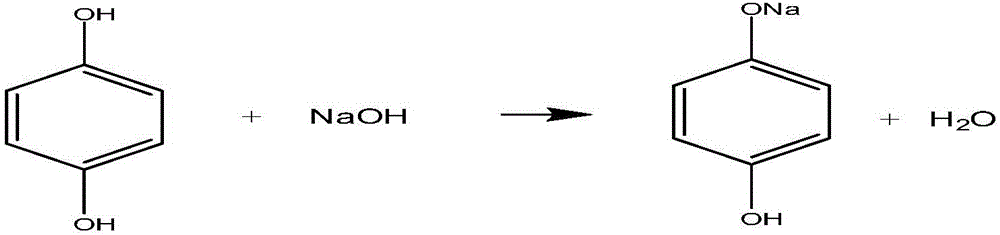Synthesizing method for (R)-(+)-2-(4-hydroxy phenoxy) methyl propionate
A technology of hydroxyphenoxy and methyl propionate, which is applied in the field of synthesis of pesticide intermediates, can solve the problems of reducing MAQ yield and product purity, serious dust pollution, and prolonging the synthesis cycle, so as to shorten the synthesis cycle and improve work Environment, the effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthetic method of (R)-(+)-2-(4-hydroxyphenoxy) methyl propionate described in this embodiment comprises the following steps:
[0022] (1) Put hydroquinone and sodium hydroxide aqueous solution into the reaction kettle to react to generate a mixed solution containing sodium p-hydroxyphenolate; the reaction temperature is controlled at 25°C; the mass concentration of the hydroquinone is not less than 99.5 %, the mass concentration of sodium hydroxide aqueous solution is 30%;
[0023] (2) Add methyl 2-chloropropionate to carry out substitution reaction in reactor, generate the mixed solution containing (R)-(+)-2-(4-hydroxyphenoxy) methyl propionate; Substitution reaction temperature Controlled at 25°C;
[0024] (3) add extraction solvent in reaction kettle and carry out extraction layering treatment, then steam out redundant extraction solvent through oil layer distillation; Described extraction solvent is toluene, and the mass concentration of toluene is not less t...
Embodiment 2
[0028] The synthetic method of (R)-(+)-2-(4-hydroxyphenoxy) methyl propionate described in this embodiment comprises the following steps:
[0029] (1) Put hydroquinone and sodium hydroxide aqueous solution into the reaction kettle to react to generate a mixed solution containing sodium p-hydroxyphenolate; the reaction temperature is controlled at 30° C.; the mass concentration of the hydroquinone is not less than 99.5 %, the mass concentration of aqueous sodium hydroxide solution is 29%;
[0030] (2) Add methyl 2-chloropropionate to carry out substitution reaction in reactor, generate the mixed solution containing (R)-(+)-2-(4-hydroxyphenoxy) methyl propionate; Substitution reaction temperature Controlled at 30°C;
[0031] (3) add extraction solvent in reaction kettle and carry out extraction layering treatment, then steam out redundant extraction solvent through oil layer distillation; Described extraction solvent is toluene, and the mass concentration of toluene is not less...
Embodiment 3
[0035] The synthetic method of (R)-(+)-2-(4-hydroxyphenoxy) methyl propionate described in this embodiment comprises the following steps:
[0036] (1) Put hydroquinone and sodium hydroxide aqueous solution into the reaction kettle to react to generate a mixed solution containing sodium p-hydroxyphenolate; the reaction temperature is controlled at 27°C; the mass concentration of the hydroquinone is not less than 99.5 %, the mass concentration of sodium hydroxide aqueous solution is 35%;
[0037] (2) Add methyl 2-chloropropionate to carry out substitution reaction in reactor, generate the mixed solution containing (R)-(+)-2-(4-hydroxyphenoxy) methyl propionate; Substitution reaction temperature Controlled at 26°C;
[0038] (3) add extraction solvent in reaction kettle and carry out extraction layering treatment, then steam out redundant extraction solvent through oil layer distillation; Described extraction solvent is toluene, and the mass concentration of toluene is not less t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com