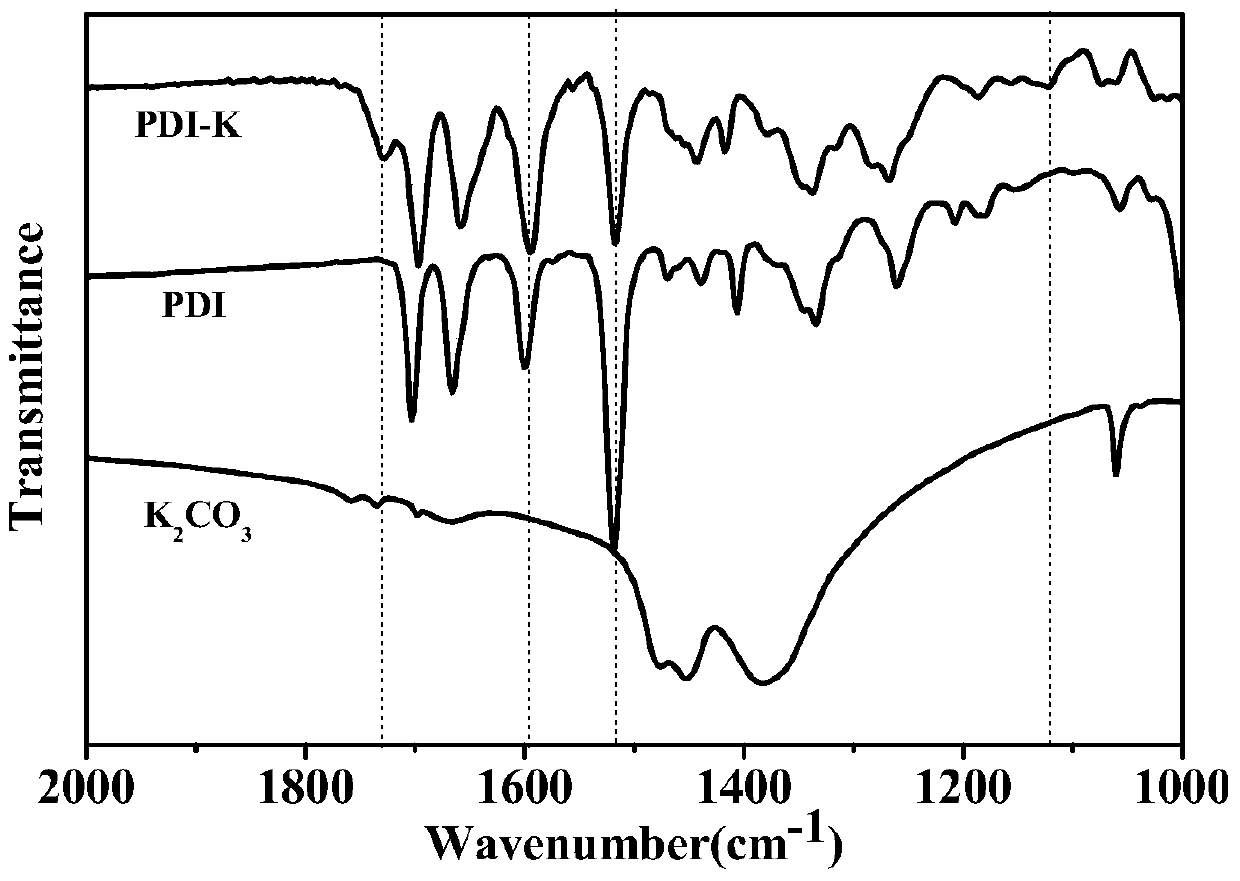
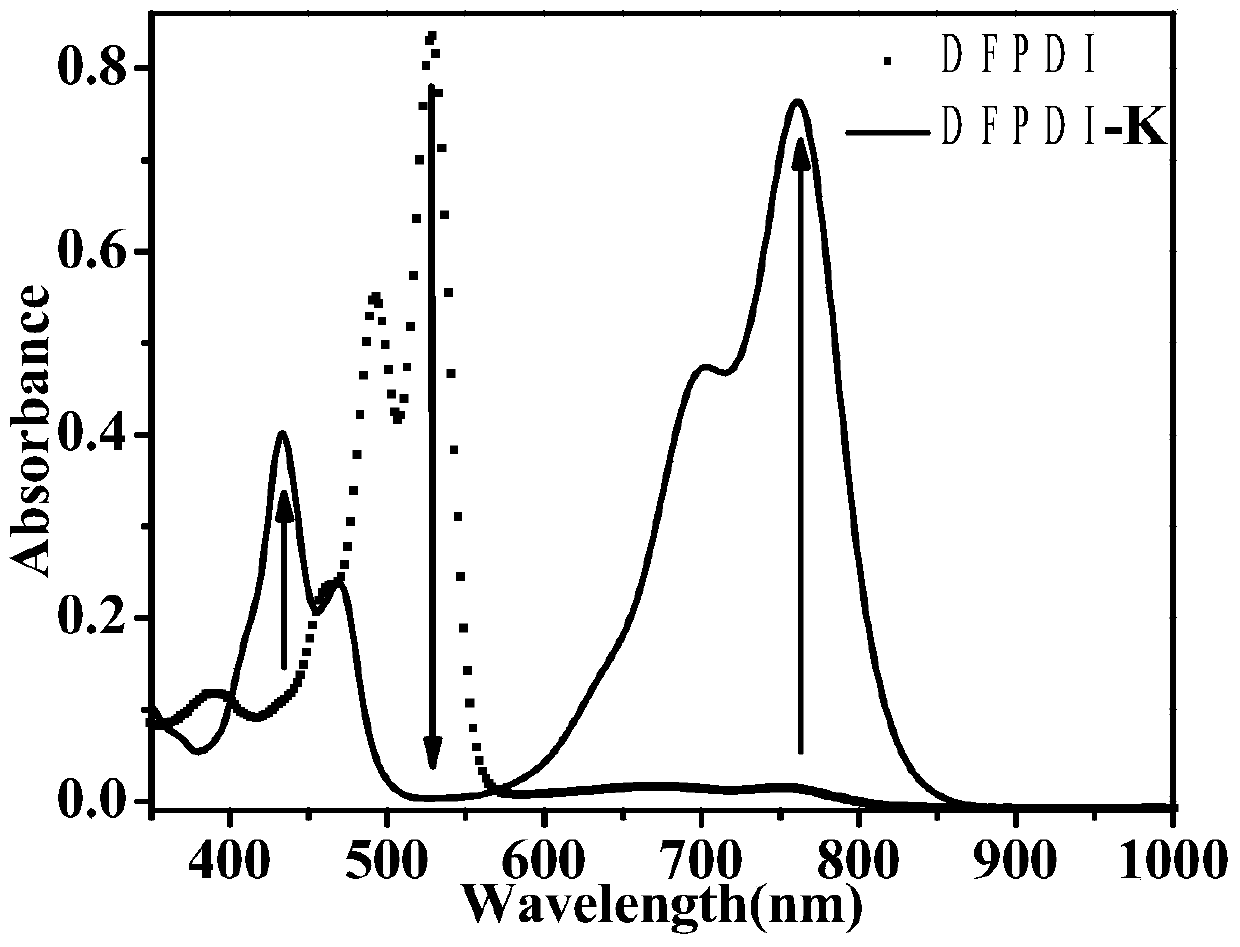
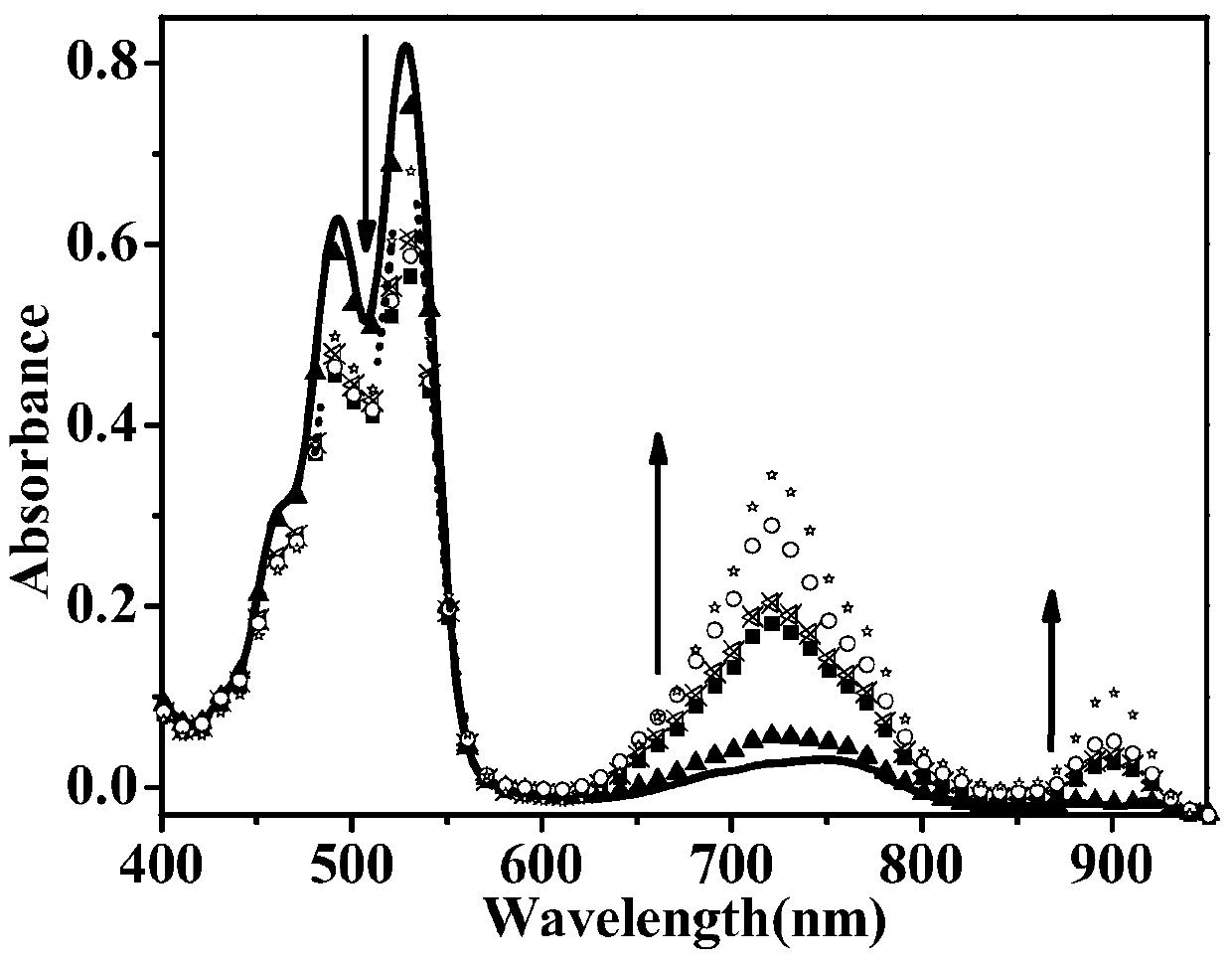
Preparation method of reduced ionic salt of peryleneimide and its derivatives
A technology of perylene imide and derivatives, applied in the field of organic compounds and their synthesis, can solve the problems of harsh requirements, unsuitable industrialization, high cost, and achieve the effects of low price, easy availability of raw materials and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Add 2.14g of 3,4,9,10-perylenetetracarboxylic dianhydride (PTDA) (M=392) to the 18mL concentrated (98%) H 2 SO 4 (M=98,1.836g / mL) in a three-necked flask, vibrate with 35Hz ultrasonic for 10min, then place it on a heat-collecting thermostatic magnetic stirrer and add a condenser. After stirring at room temperature for 6h, adjust the temperature to 80 ℃, reflux reaction, during which 0.053g I was added 2 (M=127) As a catalyst, about 0.577 mL (3.12 g / mL) of liquid bromine (M=160) was slowly added dropwise into it using a constant pressure dropping funnel. After 24 hours of reaction, the reaction solution was diluted with distilled water to 60% concentrated sulfuric acid and filtered with suction to obtain a filter cake. The filter cake was dried in a vacuum drying oven at 90°C for 24 hours. The filter cake mainly contains the aforementioned three compounds a, b, and c, where a:b:c=7:2:1.
[0049] In a 100ml three-necked reaction flask, 0.89g of bromoperylenetetracarboxylic a...
Embodiment 2
[0054] Add 2.09g of 3,4,9,10-perylenetetracarboxylic dianhydride (PTDA) (M=392) to the 18mL concentrated (98%) H 2 SO 4 (M=98,1.836g / mL) in a three-necked flask, vibrate with 35Hz ultrasonic for 10min, then place it on a heat-collecting thermostatic magnetic stirrer and add a condenser. After stirring at room temperature for 6h, adjust the temperature to 80 ℃, reflux reaction, during which 0.052g I was added 2 (M=127) As a catalyst, about 0.58 mL (3.12 g / mL) of liquid bromine (M=160) was slowly added dropwise into it using a constant pressure dropping funnel. After 24 hours of reaction, the reaction solution was diluted with distilled water to 60% concentrated sulfuric acid and filtered with suction to obtain a filter cake. The filter cake was dried in a vacuum drying oven at 90°C for 24 hours. The filter cake mainly contains the aforementioned three compounds a, b, and c, where a:b:c=7:2:1.
[0055] In a 100ml three-necked reaction flask, add 0.91g of bromoperylenetetracarboxyli...
Embodiment 3
[0060] Add 1.98g of 3,4,9,10-perylenetetracarboxylic dianhydride (PTDA) to 18mL of concentrated (98%) H 2 SO 4 In a three-necked flask with 35Hz ultrasonic vibration for 10min, place it on a heat-collecting thermostatic magnetic stirrer and add a condensing device. After stirring for 6h at room temperature, adjust the temperature to 80℃, reflux reaction, and add 0.052g I 2 As a catalyst, about 0.58 mL of liquid bromine was slowly added dropwise into it using a constant pressure dropping funnel. After 24 hours of reaction, the reaction solution was diluted with distilled water to 60% concentrated sulfuric acid and filtered with suction to obtain a filter cake. The filter cake was dried in a vacuum drying oven at 90°C for 24 hours. The filter cake mainly contains the aforementioned three compounds a, b, and c, where a:b:c=7:2:1.
[0061] In a 100ml three-necked reaction flask, add 0.95g of bromoperylenetetracarboxylic anhydride prepared by the above steps, 0.52mL of 17.5mol / L cataly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com