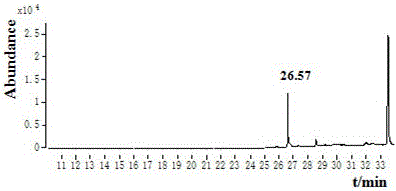
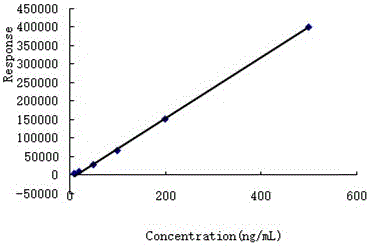
GC-EI-MS method for rapidly determining residual ametoctradin amount
A technology of GC-EI-MS and oxflufen, which is applied in the field of GC-EI-MS rapid determination of oxflufen residues, can solve the problems of high price, long system, and no visibility, and avoids the need for Matrix interference, good reproducibility, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Detection of fenflufenoxam residue in pork
[0033] (1) Sample pretreatment
[0034] extract
[0035] Weigh a well-mixed 5g pork sample into a 50mL centrifuge tube, add 10mL water to resuscitate for 30 minutes, add 20mL acetonitrile solution, shake and extract for 20min, ultrasonically extract for 5min, add 3g anhydrous magnesium sulfate and 2g sodium chloride, vortex After 1 min, centrifuge at 7000r / min for 5 min and wait for purification.
[0036] Purify
[0037] Add 4mL of water to the purification tube containing the enhanced lipid removal product EMR, vortex to fully activate it, pipette 6mL sample extract into the activated EMR purification tube, vortex for 1min, centrifuge at 7000r / min for 5min , Transfer all the supernatant to a centrifuge tube containing anhydrous magnesium sulfate and sodium chloride for salting out, after vortex centrifugation, pipette 4mL supernatant, concentrate to dryness, use a volume ratio of 1 / 1 Acetone / n-hexane mixed solvent is dis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com