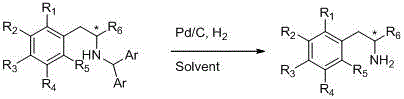Chiral beta-arylamine compounds prepared by asymmetric reductive amination reaction and preparation method of chiral beta-arylamine compounds
A technology of amination reaction and aryl amines, which is applied in the field of chiral β-aryl amines, can solve the problems of high cost, low reaction yield, slow development of asymmetric reductive amination, etc., and achieves fewer steps , high catalytic efficiency, simple and cheap reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] The present invention will be described in detail below in combination with specific embodiments.
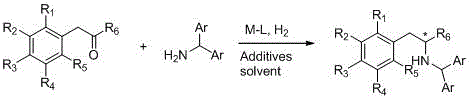
[0039] The synthesis method of chiral β-arylamine compounds involved in the present invention is based on the asymmetric reductive amination reaction, using α-aryl ketone and diarylmethylamine to hydrogenate under the action of metal iridium or rhodium catalyst, and obtains in one step The target product is chiral β-arylamine compound.
[0040] The specific reaction is as follows:
[0041]
[0042] in:
[0043] M is a salt of metal iridium or metal rhodium;
[0044] L is a chiral monophosphine, diphosphine or phosphine nitrogen ligand;
[0045] Additives is a combination of additives;
[0046] Solvent is the reaction solvent;
[0047] R 6 is an alkyl, aryl, ester, amide or carboxylic acid group;
[0048] R 1 , R 2 , R 3 , R 4 , R 5 are the same or different hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, unsaturated monocyclic hydrocarbon, alkoxy, halogen, h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com