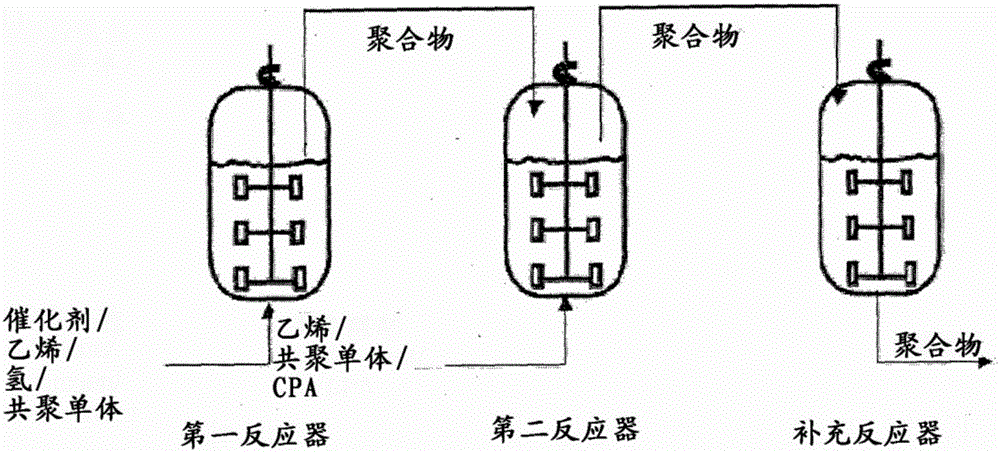
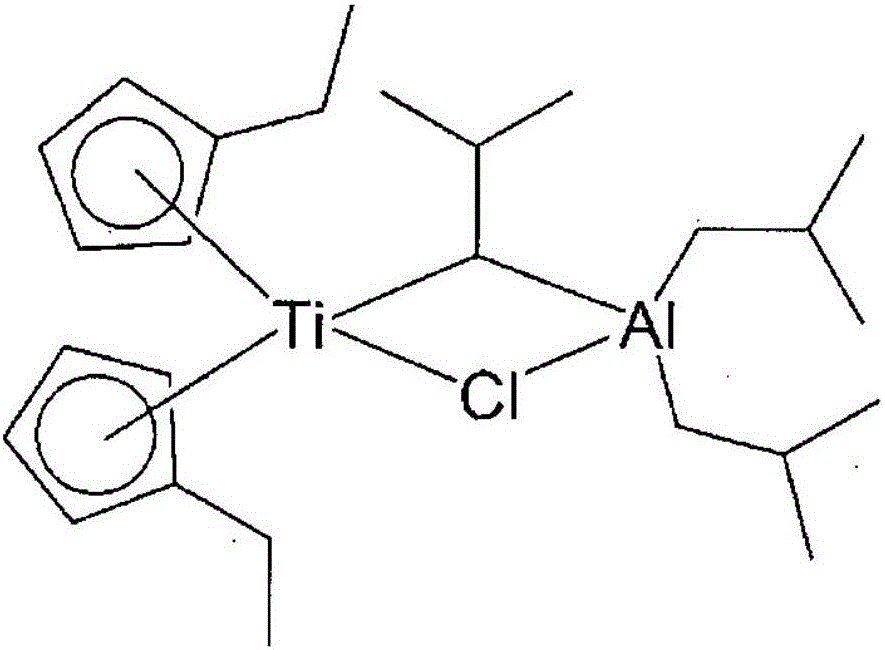
Method for preparing polyolefin and polyolefin prepared thereby
A technology for polyolefins and olefin monomers, applied in the field of polyolefins, can solve the problems of poor processability, uneven surface, and reduced bubble stability of polyolefins.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0178] [t-Bu-O(CH 2 ) 6 -C 5 H 4 ] 2 ZrCl 2 Synthesis
[0179] The preparation of tert-butyl-O-(CH 2 ) 6 -Cl, and make it with NaC 5 h 5 reaction to obtain tert-butyl-O-(CH 2 ) 6 -C 5 h 5 (Yield: 60%, b.p. 80°C / 0.1 mmHg).
[0180] At -78°C, 2.0 g (9.0 mmol) tert-butyl-O-(CH 2 ) 6 -C 5 h 5 It was dissolved in THF, and 1.0 equivalent of n-butyllithium (n-BuLi) was slowly added thereto. The temperature was raised to room temperature, and the reaction was allowed to proceed for 8 hours. At -78 °C, the reaction solution was slowly added to the ZrCl 4 (THF) 2(1.70 g, 4.50 mmol) / THF (30 mL) suspension, and then further reacted at room temperature for 6 hours to obtain a final reaction solution.
[0181] The reaction solution was dried under vacuum to remove all volatile materials, then hexane was added to the remaining oily liquid, followed by filtration using a Schlenk glass filter. The filtrate was dried under vacuum to remove the solvent...
Synthetic example 2
[0185]
[0186] 2-1. Preparation of Ligand Compounds
[0187] 2 g of fluorene was dissolved in 5 mL of MTBE, 100 mL of hexane, and then 5.5 mL of a 2.5 M n-BuLi hexane solution was added dropwise thereto in a dry ice / acetone bath, followed by stirring overnight at room temperature. Dissolve 3.6 g of (6-(tert-butoxy)hexyl)dichloro(methyl)silane in 50 mL of hexane, and transfer the fluorene-Li slurry in a dry ice / acetone bath for 30 min, followed by stirring overnight at room temperature . Simultaneously, 5,8-dimethyl-5,10-dihydroindeno[1,2-b]indole (12mmol, 2.8g) was dissolved in 60mL THF and poured into it gradually in a dry ice / acetone bath 5.5 mL of 2.5M n-BuLi hexane solution was added dropwise, followed by stirring overnight at room temperature. NMR sampling of the reaction solution of fluorene and (6-(tert-butoxy)hexyl)dichloro(methyl)silane was performed to verify the completion of the reaction, and then 5,8-dimethyl-5 , 10-dihydroindeno[1,2-b]indole-Li solution ...
Synthetic example 3
[0193]
[0194] 3-1. Preparation of Ligand Compounds
[0195] 2.1 g (9 mmol) of 5,8-dimethyl-5,10-dihydroindeno[1,2-b]indole was added to a 250 mL flask in which the atmosphere had been replaced with an argon atmosphere, and dissolved in 50 mL of THF . Thereto was added dropwise 3.9 mL (9.75 mmol) of a 2.5 M n-BuLi hexane solution in a dry ice / acetone bath, followed by stirring overnight at room temperature. Thus, a yellow slurry was obtained. A further 50 ml of hexane was injected, and 1.35 g of (6-(tert-butoxy)hexyl)dichloro(methyl)silane was added dropwise using a syringe in a dry ice / acetone bath, and the temperature was raised to room temperature, followed by stirring overnight . A small amount of reaction product was sampled to verify completion of the reaction by 1H-NMR. The solvent was dried under vacuum, then the resulting solid was dissolved in 70 ml toluene and filtered to remove LiCl. The resulting filtrate was used as such for metallization.
[0196] 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt flow index | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com