Aliphatic polycarbonate dibasic alcohol and preparation method thereof
A technology of aliphatic diol and polycarbonate diol, which is applied in the field of aliphatic polycarbonate diol and its preparation, and achieves the effects of easy impurity removal, good effect, and no solvent needed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
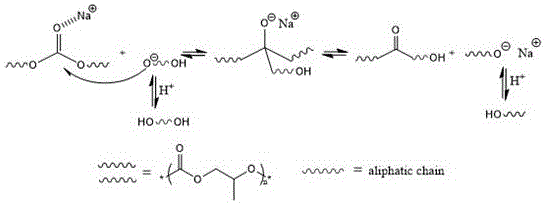
[0033] Add 10.2g (100mmol) of polymethylethylene carbonate, 0.21g of sodium carbonate (2%), and 2.51g (33mmol) of 1,2-propanediol into a heating and stirring system, temperature measuring system and reflux system inside the reactor. Repeatedly evacuate and ventilate the nitrogen to remove the air in the reactor (at least 3 times), and raise the temperature to 180°C at normal pressure under the nitrogen atmosphere, and react for 1h. Nitrogen gas is passed to allow the product to cool to obtain the primary product of polymethylethylene carbonate diol. The obtained primary product is a slightly yellow viscous liquid at room temperature, which can be washed 3 times with water, and the by-products can be removed after soaking, and the color turns white. Then azeotropic distillation with toluene removes the water therein, and the polymethylethylene carbonate diol is obtained after drying.
[0034] Product parameters:
[0035]
Embodiment 2
[0037] Add 10.2g (100mmol) of polymethylethylene carbonate, 53mg of sodium carbonate (0.5%), and 1.50g (16.67mmol) of 1,4-butanediol into a heating and stirring system, temperature measuring system and reflux in the reactor of the system. Repeatedly evacuate and ventilate the nitrogen to remove the air in the reactor (at least 3 times), raise the temperature to 150°C at normal pressure under nitrogen atmosphere, and react for 3 hours. Nitrogen gas is passed to allow the product to cool to obtain the primary product of polymethylethylene carbonate diol. The obtained primary product is a slightly yellow viscous liquid at room temperature, which can be washed 3 times with water, and the by-products can be removed after soaking, and the color turns white. Then azeotropic distillation with toluene removes the water therein, and the polymethylethylene carbonate diol is obtained after drying.
[0038] Product parameters:
[0039]
Embodiment 3
[0041] Add 10.2g (100mmol) of polymethylethylene carbonate, 0.42g of sodium bicarbonate (5.0%), and 11.80g (100mmol) of 1,6-hexanediol into a heating and stirring system, temperature measuring system and In the reactor of the reflux system. Repeatedly evacuate the nitrogen to remove the air in the reactor (at least 3 times), raise the temperature to 100°C under the nitrogen atmosphere, and react for 20h. Nitrogen gas is allowed to cool the product to obtain polymethylethylene carbonate diol. The obtained primary product is a slightly yellow viscous liquid at room temperature, which can be washed 3 times with water, and the by-products can be removed after soaking, and the color turns white. Then azeotropic distillation with toluene removes the water therein, and the polymethylethylene carbonate diol is obtained after drying.
[0042] Product parameters:
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com