Sodium ion battery positive electrode material, and preparation method and application method thereof
A technology for sodium ion batteries and cathode materials, applied in the field of electrochemistry, can solve the problems of poor reproducibility of precursor composition control, uncontrollable product structure and morphology, etc., and achieve the effect of structure optimization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
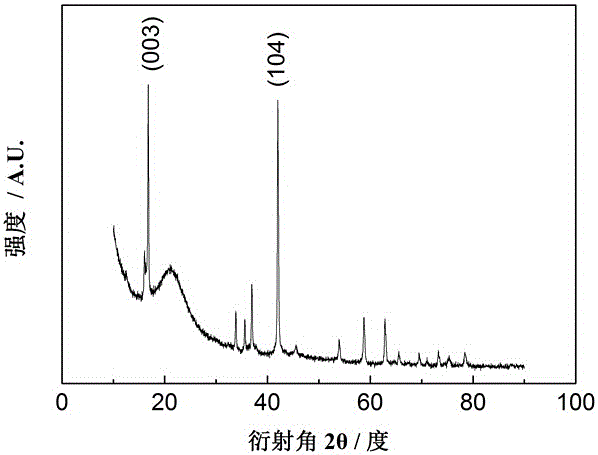
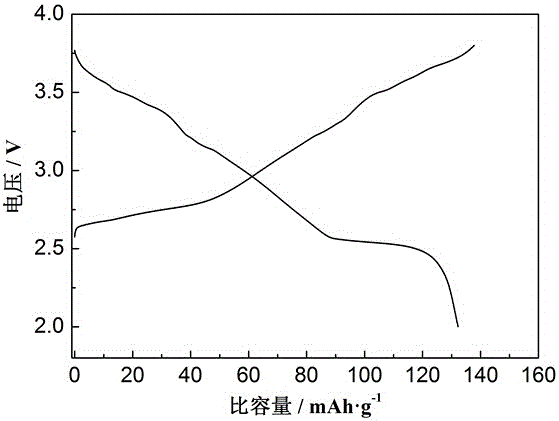
Image
Examples
Embodiment 1
[0034] 1) The reactants and solvents are all commercial reagents, and 28.52g of aqueous nickel chloride (NiCl 2 ·6H 2O) and 15.83g of aqueous manganese chloride (MnCl 2 4H 2 O) Transfer to 100mL deionized water to prepare an aqueous solution with a total cation concentration of 2mol / L and heat it to 60-80°C, and adjust the pH value of the aqueous solution to 7 with an appropriate amount of ammonia water; prepare 400mL of ammonium oxalate solution with a concentration of 0.5mol / L, Add 2.52 g of oxalic acid to the ammonium oxalate solution; after the preparation is completed, slowly add the above-mentioned ammonium oxalate solution dropwise to the heated and stirred mixed salt solution, and quickly add the ammonium oxalate solution when it becomes turbid, and filter to obtain the precipitate;
[0035] 2) Mix the precipitate with 13.40g of sodium oxalate and transfer it to a hydrothermal reaction device. The hydrothermal solvent is 70g of a mixture of ethanol and water with a m...
Embodiment 2
[0042] 1) Both reactants and solvents use commercial reagents, 26.28g of nickel sulfate hexahydrate and 15.1g of manganese sulfate (MnSO 4 ) was transferred to 200mL deionized water to prepare an aqueous solution with a total cation concentration of 1mol / L and heated to 60°C, and adjusted the pH of the aqueous solution to 6 with an appropriate amount of ammonia water; prepared 200mL of ammonium oxalate solution with a concentration of 1mol / L, and added An additional 5.04 g of oxalic acid was added to the solution; after the preparation was completed, slowly add the above-mentioned ammonium oxalate solution dropwise to the mixed salt solution under heating and stirring, and quickly add the ammonium oxalate solution when turbidity appeared, and filter to obtain the precipitate;
[0043] 2) Mix the precipitate with 8.0g of sodium hydroxide and transfer it to a hydrothermal reaction device. The hydrothermal solvent is 40g of a mixture of ethanol and water with a mass ratio of 1:1. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com