Compound antihypertensive drug combination preparation containing furosemide and spirolactone and preparation method of compound antihypertensive drug combination preparation
A technology of furosemide and spironolactone, which is applied to the compound antihypertensive drug combination preparation containing furosemide and spironolactone and the field of preparation thereof, can solve the problems of low bioavailability, etc. The effect of increased surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
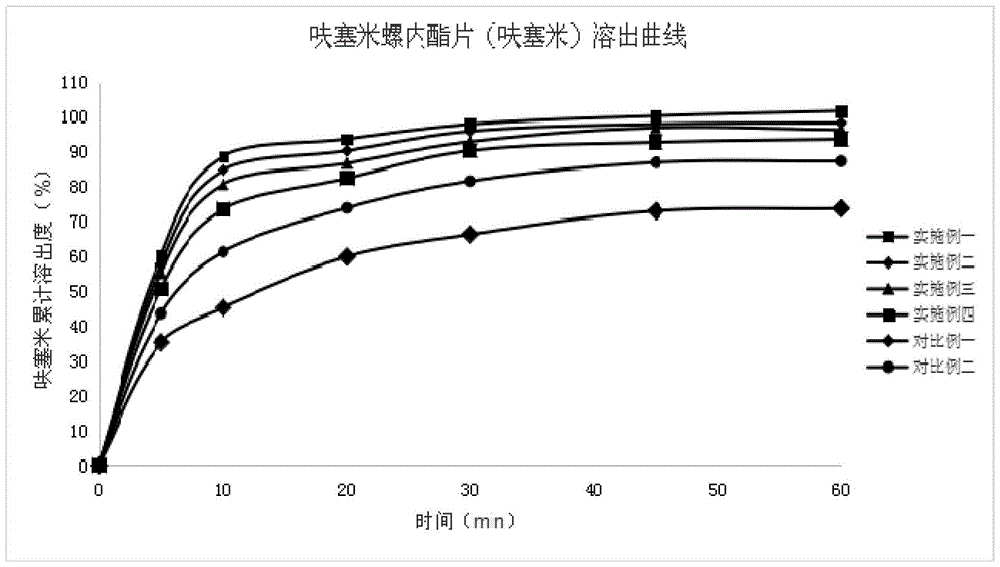
Image
Examples
preparation example Construction
[0036] According to a typical embodiment of the present invention, a preparation method of the above compound antihypertensive drug combination preparation is provided. The preparation method comprises the following steps: S1, respectively superfinely pulverizing furosemide and spironolactone so that their particle diameters D90 are respectively below 12 μm, and uniformly mixing the pulverized furosemide and spironolactone to obtain mixed powder A; S2, Mix mixed powder A with filler, 60% disintegrant and solubilizer evenly, add binder and appropriate amount of solvent to prepare soft material, then granulate and dry; S3, the granules after step S2 are dried After granulation, add the remaining disintegrant and lubricant, mix evenly, and compress the tablet to obtain the tablet core; S4, coat the tablet core, and obtain the compound antihypertensive drug combination preparation after drying.
[0037]In the present invention, the crude drug is ultrafinely pulverized so that its ...
Embodiment 1
[0043]
[0044] Coating solution:
[0045]
[0046] Preparation Process:
[0047] ①Ultrafinely pulverize furosemide and spironolactone, the particle size D90 of which is less than 12 μm, then mix evenly and set aside;
[0048] ②Weigh the prescribed amount of lactose, microcrystalline cellulose, poloxamer and 7.2g of low-substituted hydroxypropyl cellulose and mix them with the two main ingredients evenly, add 50% ethanol solution to make soft material, and pass through a 20-mesh sieve to granulate ;
[0049] ③ Dry the wet granules in an oven at 60°C for 1.5 to 2 hours, and take the dried granules and pass them through a 20-mesh sieve for granulation;
[0050] ④Take the dry granules, add the prescribed amount of magnesium stearate and 4.8g of low-substituted hydroxypropyl cellulose, mix well, and punch the tablets with 8mm shallow concaves to obtain plain tablets;
[0051] ⑤Place the beaker with 50% ethanol solution on a magnetic stirrer, slowly add Opadry (295F620027)...
Embodiment 2
[0054]
[0055] Coating solution:
[0056]
[0057]
[0058] Preparation Process:
[0059] ① Pulverize furosemide and spironolactone, the particle size D90 of which is less than 12 μm, then mix evenly and set aside;
[0060] ② Weigh the prescription amount of lactose, microcrystalline cellulose, Tween 80 and 6.0 g of prescription amount of carboxymethyl starch sodium and mix with the two main ingredients, add water to make soft material, and pass through a 20-mesh sieve to granulate;
[0061] ③ Dry the wet granules in an oven at 60°C for 1.5 to 2 hours, and take the dried granules and pass them through a 20-mesh sieve for granulation;
[0062] ④Take the dry granules, add the prescribed amount of magnesium stearate and 4.0 g of sodium starch glycolate, mix well, and punch the tablets with 8 mm shallow concaves to obtain plain tablets;
[0063] ⑤Place the beaker with 50% ethanol solution on a magnetic stirrer, slowly add Opadry (295F620027) into the constantly stirrin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com