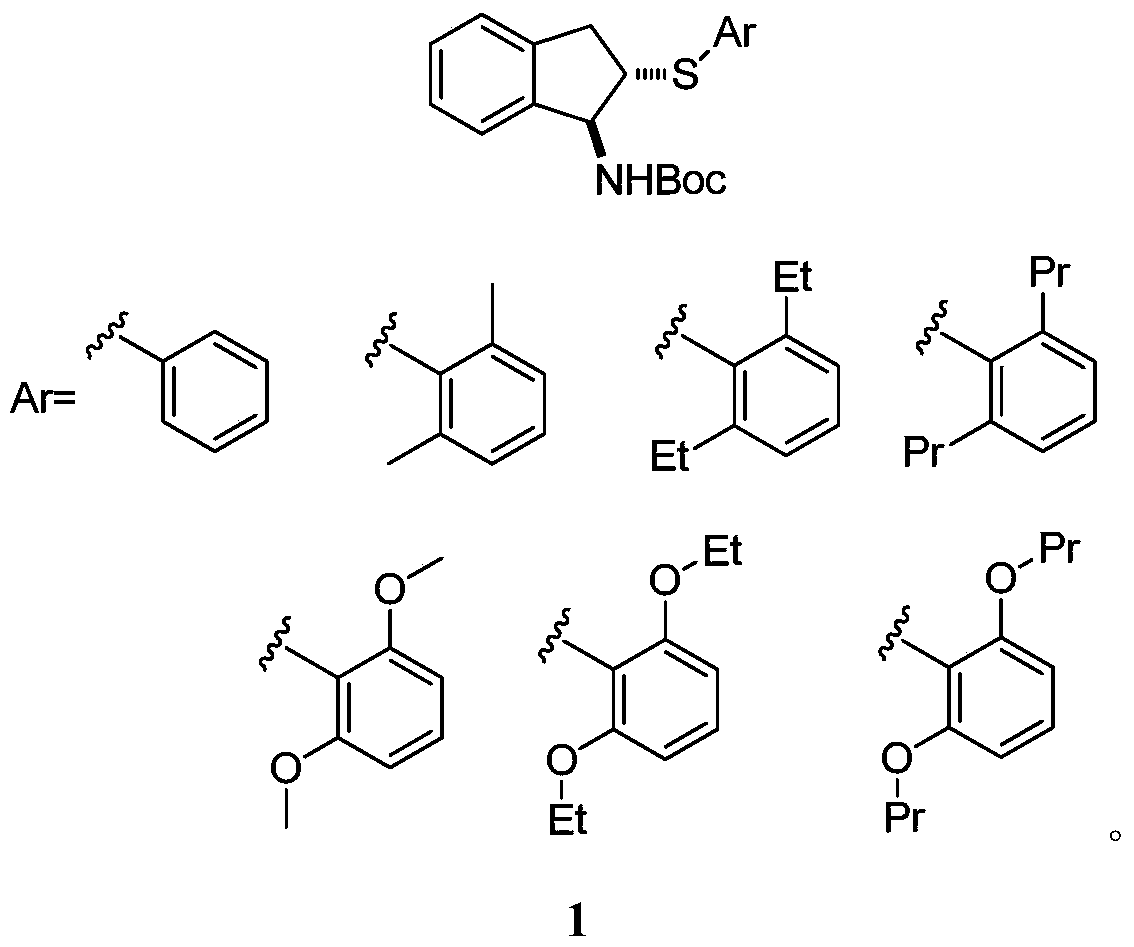
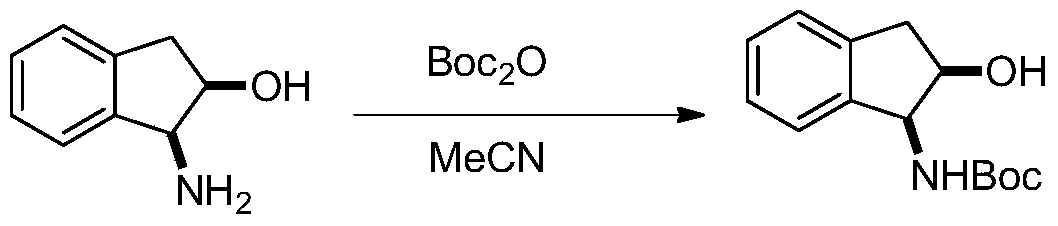Chiral organic selenium sulfur catalyst and its preparation method and application in asymmetric reaction
A technology of organic selenium and catalyst, which is applied in the field of chiral organic selenium sulfur catalysis, can solve the problems of inability to complete the asymmetric trifluoromethylthio esterification reaction of alkenes, and achieve excellent optical selectivity, convenient operation, and enantioselectivity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Put 1.34g of (1S,2R)-(+)-1-amino-2-indanol in a 100mL reaction flask, vacuumize and fill with nitrogen, and repeat this process three times. Then place the reaction bottle in an ice bath and cool it down to 0°C, then add 2.55g Boc 2 0 and 36 mL of redistilled acetonitrile were added to the reactor, and the ice bath was removed and stirred for 5 hours until the solution was clear and transparent, indicating that the reaction had been completed. Spin-dry directly to obtain a viscous liquid, and proceed directly to the next step. The synthetic formula of this step is:
[0020]
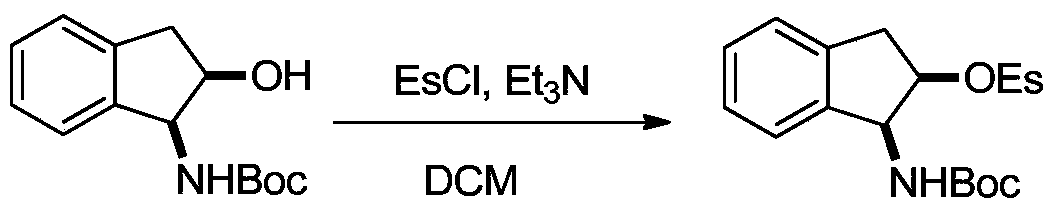
[0021] (2) Place the Boc-protected indenaminol prepared above in a 100 mL reaction flask, first vacuumize and fill with nitrogen, and repeat this three times. After adding 30 mL of redistilled dichloromethane, the reaction bottle was cooled to 0°C in an ice bath, and then 2 mL of triethylamine and 1 mL of ethylsulfonyl chloride were added thereto. Allow the ice bath to slowly return to ro...
Embodiment 2
[0029] (1) Put 1.34g of (1S,2R)-(+)-1-amino-2-indanol in a 100mL reaction flask, vacuumize and fill with nitrogen, and repeat this process three times. Then place the reaction bottle in an ice bath and cool it down to 0°C, then add 2.55g Boc 2 0 and 36 mL of redistilled acetonitrile were added to the reactor, and the ice bath was removed and stirred for 5 hours until the solution was clear and transparent, indicating that the reaction had been completed. Spin-dry directly to obtain a viscous liquid, and proceed directly to the next step. The synthetic formula of this step is:
[0030]
[0031] (2) Place the Boc-protected indenaminol prepared above in a 100 mL reaction flask, first vacuumize and fill with nitrogen, and repeat this three times. After adding 30 mL of redistilled dichloromethane, the reaction bottle was cooled to 0°C in an ice bath, and then 2 mL of triethylamine and 1 mL of ethylsulfonyl chloride were added thereto. Allow the ice bath to slowly return to ro...
Embodiment 3
[0038] (1) Put 1.34g of (1R,2S)-(+)-1-amino-2-indanol in a 100mL reaction flask, vacuumize and fill with nitrogen, and repeat this process three times. Then place the reaction bottle in an ice bath and cool it down to 0°C, then add 2.55g Boc 2 0 and 36 mL of redistilled acetonitrile were added to the reactor, and the ice bath was removed and stirred for 5 hours until the solution was clear and transparent, indicating that the reaction had been completed. Spin-dry directly to obtain a viscous liquid, and proceed directly to the next step. The synthetic formula of this step is:
[0039]
[0040] (2) Place the Boc-protected indenaminol prepared above in a 100 mL reaction flask, first vacuumize and fill with nitrogen, and repeat this three times. After adding 30 mL of redistilled dichloromethane, the reaction bottle was cooled to 0°C in an ice bath, and then 2 mL of triethylamine and 1 mL of ethylsulfonyl chloride were added thereto. Allow the ice bath to slowly return to ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com