Medical composition for treating climacteric syndrome and preparation thereof
A composition and syndrome technology, applied in the direction of drug combination, pharmaceutical formulation, drug delivery, etc., can solve problems such as allergies, ointment overflow, skin irritation, etc., and achieve broad application prospects, improved effects, and high safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
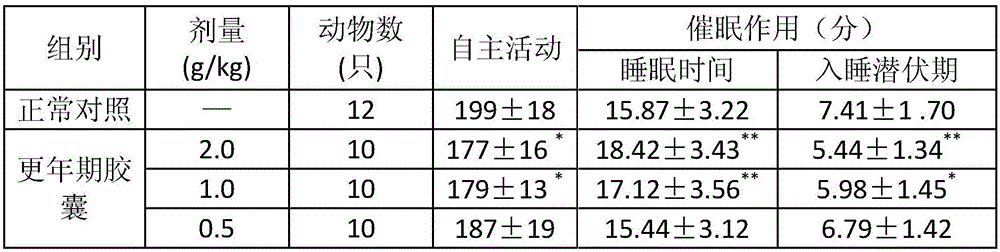
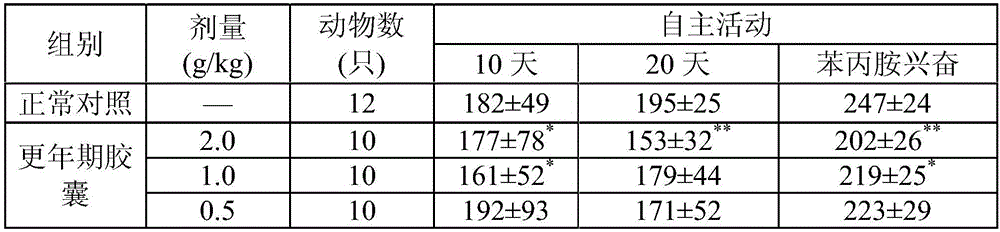
[0024] A pharmaceutical composition for treating menopausal syndrome, in terms of the following weight ratio, each component and its content are: 1 part of cassia twig, 0.5 part of dried ginger, 2 parts of schisandra, 2 parts of inula, 3.5 parts of bamboo leaves, 2 parts of peony bark, 0.5 part of jujube, 2 parts of fried jujube kernel, 2 parts of dogwood, 2 parts of raw land, 2 parts of Codonopsis pilosula, 0.5 part of gardenia. The pharmaceutical composition for treating menopausal syndrome is made into pills.
[0025] The preparation method of the pharmaceutical composition for the treatment of climacteric syndrome described above, is characterized in that: comprise the following steps,
[0026] A. The above sixteen herbs are decocted with water, the quality of the water added is 7-10 times the mass of the sixteen herbs, decocted 3 times, the first decoction is 1.5h, the second decoction is 1h, and the third decoction Cook for 0.5h;
[0027] B. merge the filtrate obtained...
Embodiment 2
[0030] A pharmaceutical composition for treating menopausal syndrome, in terms of the following weight ratio, each component and content are: 3 parts of cassia twig, 2 parts of dried ginger, 4 parts of Schisandra chinensis, 4 parts of Inula inula, 5 parts of Bamboo leaves, 4 parts of peony bark, 2 parts of jujube, 4 parts of fried jujube kernel, 4 parts of dogwood, 4 parts of raw land, 4 parts of Codonopsis pilosula, 2 parts of gardenia. The pharmaceutical composition for treating climacteric syndrome is made into an oral liquid.
[0031] The preparation method of the pharmaceutical composition for the treatment of climacteric syndrome described above, is characterized in that: comprise the following steps,
[0032] A. The above sixteen medicinal materials are decocted with water, the quality of the water added is 10 times the mass of the sixteen medicinal materials, decocted 3 times, the first decoction is 3 hours, the second decoction is 2 hours, and the third decoction is 1...
Embodiment 3
[0036]A pharmaceutical composition for treating menopausal syndrome, in terms of the following weight ratio, each component and its content are: 2.5 parts of cassia twig, 1.5 parts of dried ginger, 3.5 parts of Schisandra chinensis, 3.5 parts of Inula inula, 4.8 parts of bamboo leaves, 3.5 parts of peony bark, 1.5 parts of jujube, 3.5 parts of fried jujube kernel, 3.5 parts of dogwood, 3.5 parts of raw land, 3.5 parts of Codonopsis pilosula, 1.5 parts of gardenia. The pharmaceutical composition for treating menopausal syndrome is made into tablets.
[0037] The preparation method of the pharmaceutical composition for the treatment of climacteric syndrome described above, is characterized in that: comprise the following steps,
[0038] A. The above sixteen medicinal materials are decocted with water, the quality of the water added is 8 times the mass of the sixteen medicinal materials, decocted 3 times, the first decoction is 2 hours, the second decoction is 1.5 hours, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com