Dehydrogenation zaluzanin C derivative and preparation method and application thereof
A technology for dehydro mesothrin and derivatives, applied in the field of medicine, can solve the problem of no dehydro mesothrin C derivatives and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
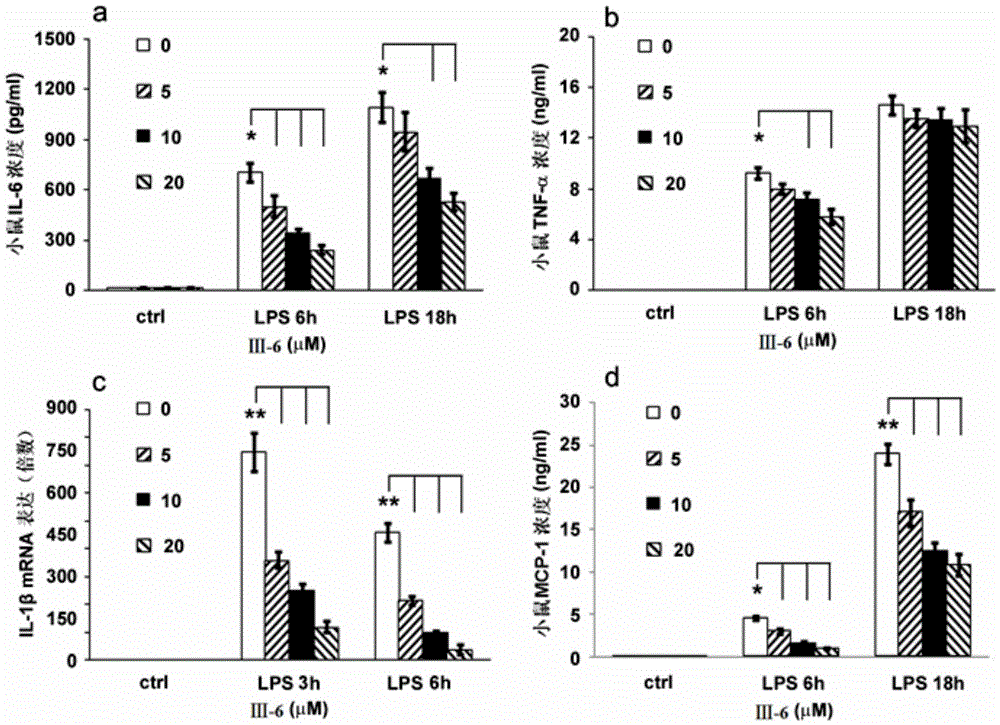
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation of compound II-5
[0046] In a 25mL double-necked flask, add dehydromethrin C (24.4mg, 0.1mmol / Lol), 4-(trifluoromethyl)piperidine (16.9mg, 0.11mmol / Lol), and then add 2mL of acetonitrile, Under the condition of stirring, triethylamine (0.11mmol / Lol, 16.0μL) was added to the reaction system, and the reaction was refluxed for 12h under the protection of argon to complete the reaction. The reaction solution was cooled to room temperature, the solvent was removed under reduced pressure, and the residue was added Dichloromethane 10mL, stirred to obtain a mixed solution, then the mixed solution was washed successively with 0.5mol / L hydrochloric acid solution, saturated sodium bicarbonate solution and saturated brine each 5mL, the organic phase was separated and dried with anhydrous sodium sulfate, filtered , and the filtrate was concentrated to obtain a crude product, which was purified by column chromatography with a mixed solvent of DCM:methan...
Embodiment 2
[0050] Embodiment 2: the preparation of compound III-6
[0051]
[0052] a) Add dehydromethrin C (24.4mg, 0.1mmol / Lol) and 5mL methanol solvent into a 25mL double-necked flask, and add NaBH to the reaction system under stirring 4 (6mg, 0.15mmol / L), stirred at room temperature for 2h under argon protection, ended the reaction, and added 1mL, 1.0mol / L NaHSO 4 The solution was quenched, and after stirring for 10 min, the organic phase was separated, the aqueous phase was extracted with ethyl acetate (4×5 mL), the organic phases were combined, washed with 5 mL of saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated , to obtain the crude product, the crude product was purified by column chromatography with a mixed solvent of petroleum ether:ethyl acetate=3:2 (volume ratio), to obtain 22.7 mg of white solid compound, i.e. intermediate III-A (molar yield was 92.3 %);
[0053] b) Add intermediate III-A (20.0mg, 0.08mmol / Lol) and 1mL py...
Embodiment 3
[0058] Embodiment 3: Preparation of Compound II-5 and Compound III-6 Tablets
[0059] After mixing each 10 g of compound II-5-HCl and compound III-6-MA in the dehydromethrin C derivative with 87.5 g (Baihujing: lactose=7:3, mass ratio), add 95 % ethanol granulation, drying, granulation (screening), adding 2.5g of sodium stearate and mixing evenly, then tableting to obtain a weight of 200 mg per tablet, the content of compound II-5-HCl or compound III-6-MA is equal to In the form of 10mg tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com