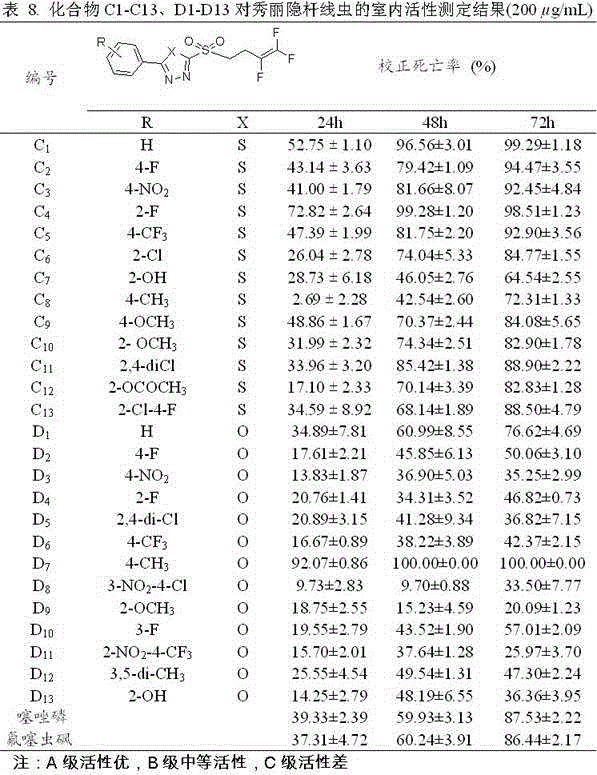
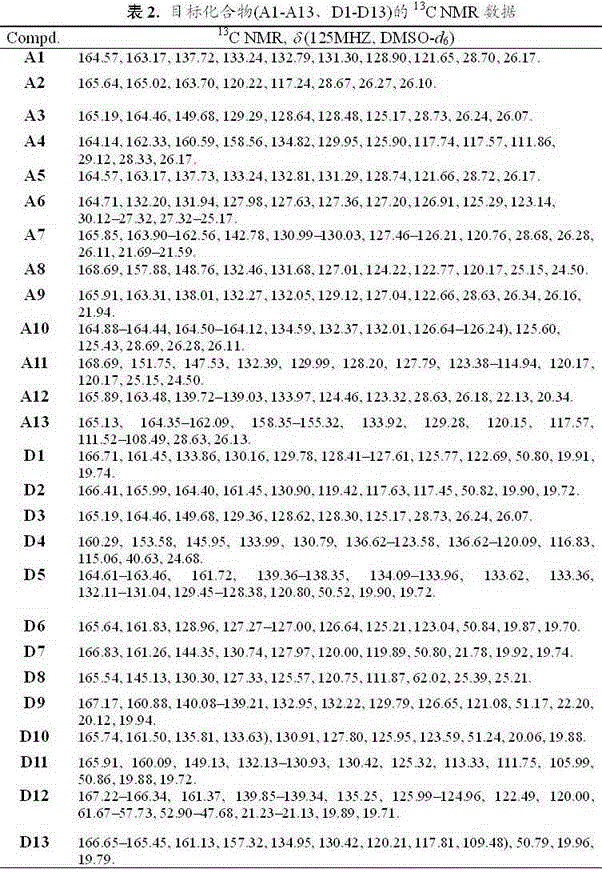
Trifluorobutenyl-containing 1,3,4-oxadiazole (thiadiazole) thioether (sulfide sulfone) derivatives and preparation method and application thereof
A technology of oxadiazole sulfide and trifluorobutene, applied in the field of chemistry, can solve the problems of crop yield reduction, commodity value reduction, loss of farmers' income and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
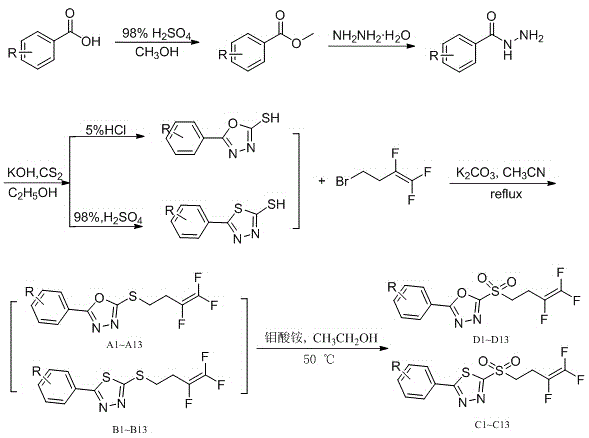
[0080] Synthesis of 2-phenyl-5-[(3,4,4-trifluoro-3-buten-1-yl)sulfur]-13,4-oxadiazole (compound number is A1):
[0081]Add 2-mercapto-5-phenyl-1,3,4-oxadiazole (0.40g, 1.53mmol) and potassium carbonate (0.25g, 1.83mmol) into a 50mL three-necked flask and add 15mL of acetonitrile, start stirring, The reaction system was a white turbid liquid, and a mixed solution of 4-bromo1,1,2-trifluoro-1-butene (0.32 g, 1.68 mmol) and 5 mL of acetonitrile was added thereto. Heated to reflux, TLC tracked the reaction process, after the raw material point disappeared, stopped the reaction, filtered out the insoluble salt, and the crude product of vacuum distillation, the crude product was recrystallized with dichloromethane to obtain the target compound, which was a white solid with a quality of 0.20g (theoretical mass of 0.23 g), the yield is 86.6%, and the melting point is 34-35°C.
Embodiment 2
[0083] Synthesis of 2-(4-fluorophenyl)-5-[(3,4,4-trifluoro-3-buten-1-yl)sulfur]-13,4-oxadiazole (Compound No. A2) :
[0084] Synthesized as in Example 1, with the difference that 2-mercapto-5-(4-fluorophenyl)-1,3,4-oxadiazole (0.60g, 3.06mmol) and 4-bromo-1, 1,2-Trifluoro-1-butene as (0.63g, 3.36mmol) to give 2-(4-fluorophenyl)-5-[(3,4,4-trifluoro-3-butene-1 -yl)sulfur]-13,4-oxadiazole, yield 77.9%, melting point 42-43°C.
Embodiment 3
[0086] Synthesis of 2-(4-nitrophenyl)-5-[(3,4,4-trifluoro-3-buten-1-yl)sulfur]-13,4-oxadiazole (compound number is A3 ):
[0087] Synthesized as in Example 1, except that 2-mercapto-5-(4-nitrophenyl)-1,3,4-oxadiazole (0.31g, 1.39mmol) and 4-bromo-1 , 1,2-Trifluoro-1-butene (0.28g, 1.53mmol) to give 2-(4-nitrophenyl)-5-[(3,4,4-trifluoro-3-butene- 1-yl)thio]-13,4-oxadiazole, yield 78.9%, melting point 84-86°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com