Method of preparing benzaldehyde from p-methyl cyclohexene formaldehyde
A technology of methylcyclohexene formaldehyde and benzaldehyde, which is applied to the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, and can solve the problems of limiting the application of benzaldehyde and long reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: catalyst preparation
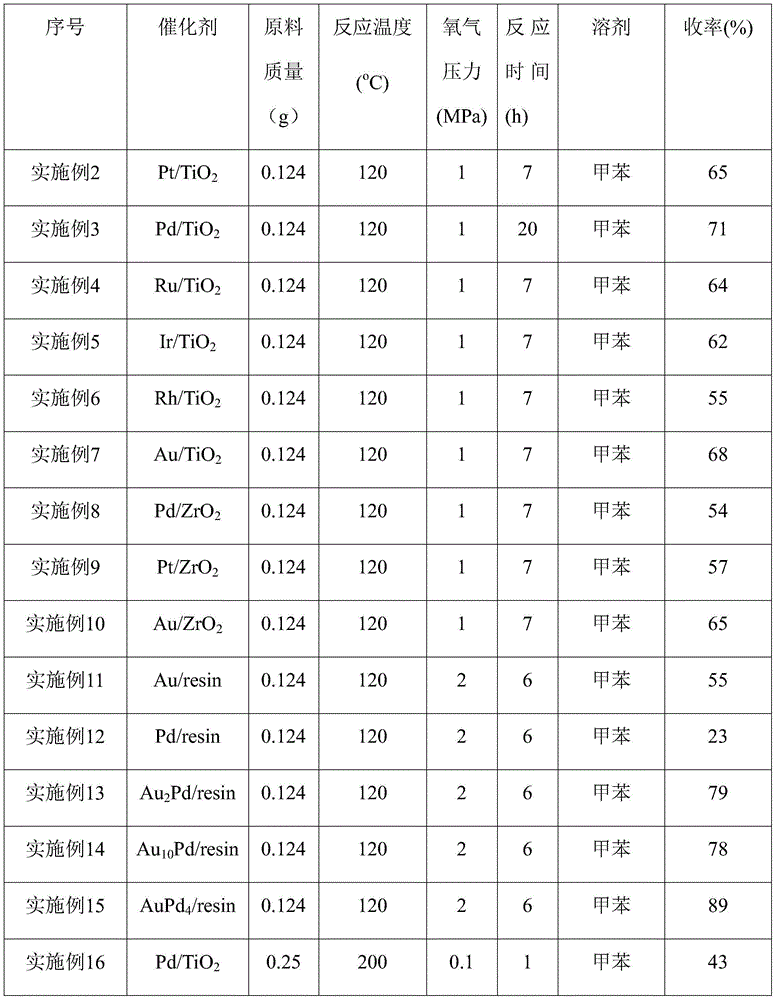
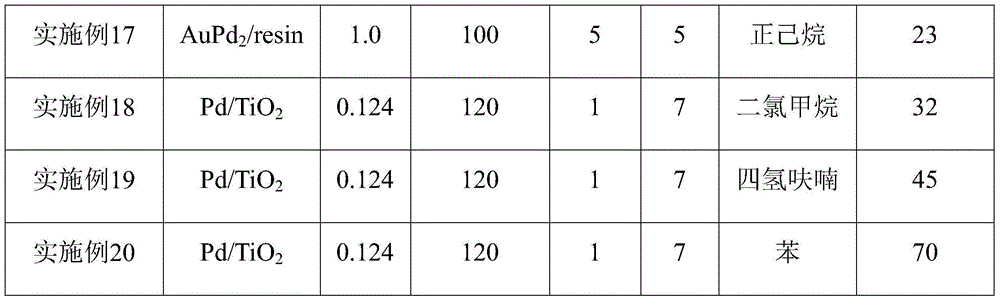
[0019] Loaded Pd / TiO 2 Catalyst preparation: Weigh 0.8374g of 20% PdCl 2 Aqueous solution, add water to a total mass of 5g, mix well and impregnate 2.0g TiO 2 (P25), after stirring, let stand for 4 hours, bake at 80°C for 1 hour, then dry overnight at 120°C, and finally reduce passivation. Reduction passivation conditions: in a hydrogen atmosphere (flow rate 120ml / min) from room temperature to 300 ° C within one hour, keep it for 1 hour and then cool down to room temperature, O 2 / N 2 Mixed gas (O 2 Volume content 1%) in passivation 4h, namely obtain 5wt%Pd / TiO 2 , to collect the catalyst for later use.
[0020] According to the above method, Pt, Ru, Ir, Rh, Pd, Au catalysts loaded on silica and zirconia were prepared respectively according to the quality of metal precursors required. The precursors of the above metal catalysts are H 2 PtCl 6 、RuCl 3 , H 2 IrCl 6 , RhCl 3 , H 2 PdCl 4 , HAuCl 4 .
[0021] Preparation...
Embodiment 2
[0028] Add 30ml of toluene, 0.124g of p-methylcyclohexene formaldehyde, 0.05g of Pd / TiO to the autoclave 2 (palladium content 1wt%), with O 2 Filled with 1MPaO after 5 times of replacement 2 , the temperature was raised to 120°C, and the reaction was carried out for 7h. After the reaction was completed, the temperature was lowered to room temperature, the reaction mixture was filtered, and the obtained filtrate was sampled and analyzed. The conversion rate and product yield were quantitatively calculated by GC-MS, and the reaction results are listed in Table 1.
Embodiment 3-10
[0030] Other reaction conditions are the same as in Example 2, and the catalysts used are 0.05 g of Pt / TiO with a loading of 1 wt%, respectively. 2 , Ru / TiO 2 , Ir / TiO 2 , Rh / TiO 2 , Au / TiO 2 , Pd / ZrO 2 , Pt / ZrO 2 , Au / ZrO 2 , and the reaction results are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com