Quality evaluation method of traditional Chinese medicines for resisting osteoporosis
An anti-osteoporosis, quality evaluation technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve problems such as quality evaluation or detection methods that have not been reported, and achieve the effect of promoting osteoblast proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Firstly, relevant biological activities of Gushukang Granules and Epimedium were measured, and the experimental results are as follows:
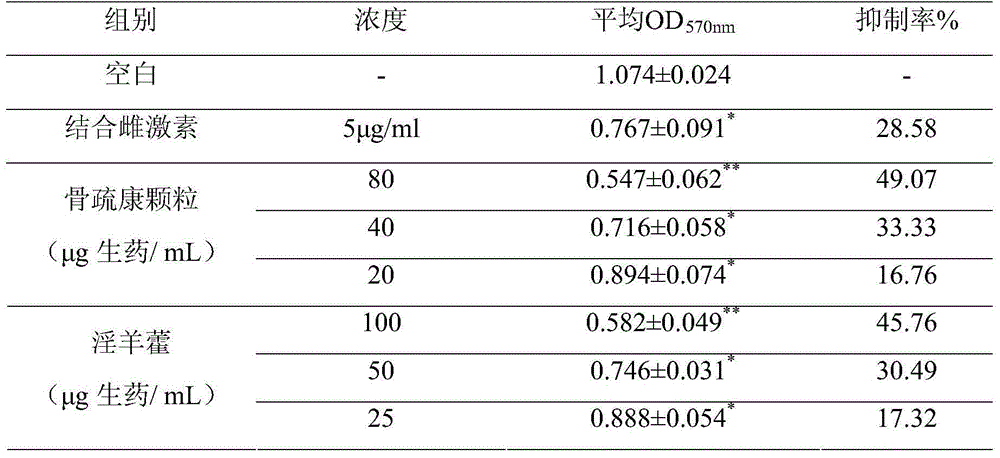
[0066] 1. Determination of the biological activity of Gushukang Granules and Epimedium in inhibiting the growth of osteoclasts by MTT method
[0067] The osteoclasts cultured in vitro were mixed with α-MEM medium containing 20% fetal bovine serum to make 1×10 5 / ml of cell suspension, seeded in 96-well plate, placed in 37 ° C, 5% CO 2 Cultivate in the incubator for 24 hours and set aside.
[0068] In the above-mentioned 96-well plate, a blank control group (no cells), a cell control group, a positive drug-conjugated estrogen group, and each dose group of the test product were set up. The blank control group and the positive drug group were respectively added with 100 μl of α-MEM culture solution to dissolve And filter-sterilized conjugated estrogen solution and 100 μl of α-MEM culture solution containing 20% fetal bovine serum, ...
Embodiment 2
[0090] Determination of the biological potency of embodiment 2 Gushukang granule inhibiting osteoclast proliferation
[0091] A. preparation of reference substance solution: take conjugated estrogen as reference substance, use the α-MEM culture fluid of 20% fetal bovine serum as solvent, make the reference substance solution that concentration is 50 μ g / ml, filter, and set aside;
[0092] B. Preparation of test solution: prepare Gushukang test solution as described above;
[0093] C. need testing solution and reference substance solution are diluted into the dosage group of different concentration with same dosage distance, and dosage distance is 0.5, and the used solvent of dilution is the α-MEM culture fluid of 20% fetal bovine serum;
[0094] D. Data processing and potency calculation: adopt the MTT method described in embodiment 1 to measure respectively the osteoclast growth inhibition rate of different dosage groups test product and reference substance, compare test prod...
Embodiment 3
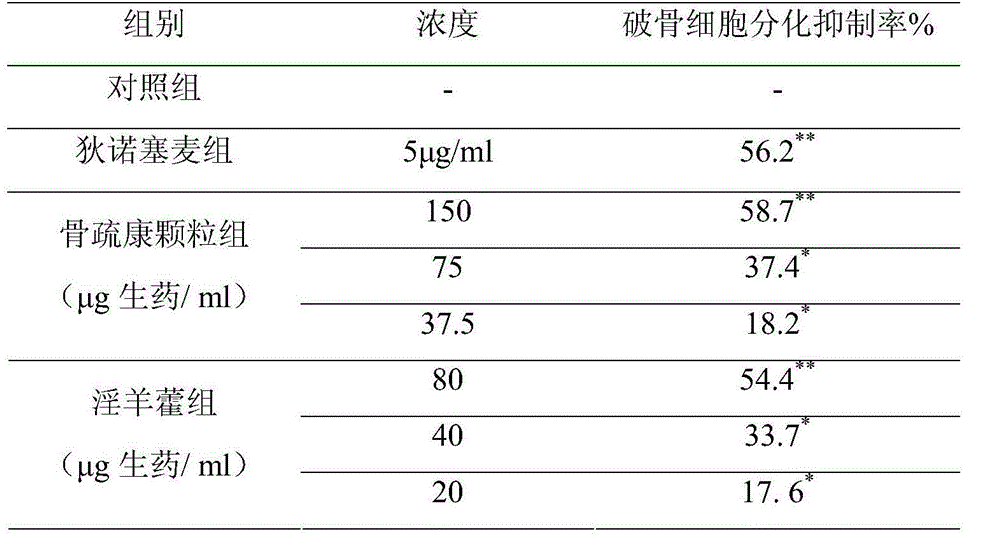
[0097] Example 3 Gushukang granule inhibits the determination of the biological potency of osteoclast differentiation
[0098] A. Preparation of reference substance solution: take denosumab as reference substance, use α-MEM culture solution of 20% fetal bovine serum as solvent, prepare a reference substance solution with a concentration of 50 μg / ml, filter, and set aside;
[0099] B. Preparation of test solution: prepare Gushukang test solution as described above;
[0100] C. need testing solution and reference substance solution are diluted into the dosage group of different concentration with same dosage distance, and dosage distance is 0.5, and the used solvent of dilution is the α-MEM culture fluid of 20% fetal calf serum;
[0101] D. Data processing and potency calculation: adopt the TRAP staining method described in embodiment 1 to measure respectively the osteoclast differentiation inhibition rate of different dosage groups test product and reference substance, compare ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com