A multi-metal catalyst for naphtha reforming and a preparing method thereof
A reforming catalyst and naphtha technology, which is applied in the direction of naphtha catalytic reforming, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of easy carbon deposition and deactivation, and achieve high isoparaffin selectivity, Good aromatization selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation method of the mesoporous γ-alumina of the present invention is as follows: first mix template agent, fatty alcohol, inorganic acid and aluminum source compound, react under the condition of pH 1-7, after drying the reactant, Roast at 300-600°C, preferably 350-450°C, preferably for 2-6 hours, remove the template agent, generate amorphous mesoporous alumina, then impregnate and introduce an appropriate amount of platinum, after drying, heat at 300-700°C, preferably 350- Mesoporous γ-alumina is obtained by calcining at 450°C, and the calcining time is preferably 2 to 12 hours.
[0026] Among the raw materials used in the method of the present invention, the molar ratio of the template agent to the aluminum source compound is preferably 1:20 to 100, and the molar ratio of the fatty alcohol to the aluminum source compound is preferably 20 to 60:1. Add the template agent to the fatty alcohol to dissolve it, Then add inorganic acid to adjust the pH value of the...
example 1
[0058] Prepare mesoporous alumina by the method of the present invention
[0059] Get the MA-400 mesoporous amorphous alumina that 20g comparative example 1 makes, impregnate 24 hours at 25 ℃ with the impregnating solution that chloroplatinic acid is made into, contain the Pt of 0.05% in the impregnating solution (relative to the quality of alumina on a dry basis ), the liquid / solid volume ratio was 2:1, filtered, the obtained solid was dried at 120°C for 8 hours, and calcined in air at 400°C for 4 hours to obtain the carrier MA-400-005, and its specific surface area is shown in Table 1.
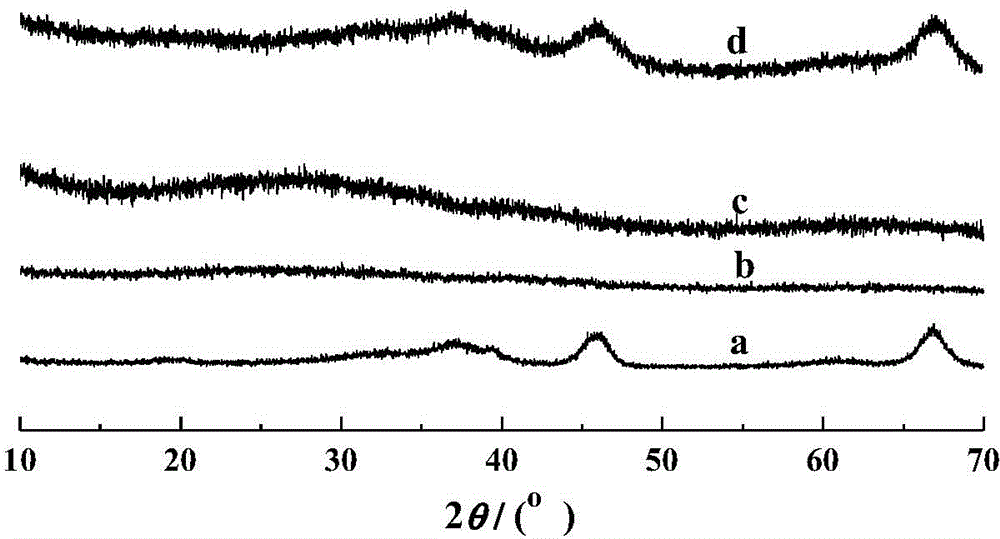
[0060] For the small angle XRD spectrum (0.5~5°) of MA-400-005 see figure 1 In the curve c, the characteristic diffraction peak of the (100) plane appears in this curve, indicating the existence of mesoporous structure.
[0061] MA-400-005 wide angle XRD spectrum (10~70°) such as image 3 As shown in the middle b curve, there are more obvious crystal diffraction peaks, indicating that the ...
example 2
[0063] Mesoporous alumina was prepared according to the method of Example 1, except that the prepared impregnation solution contained 0.10% Pt (relative to the mass of alumina on a dry basis), and the carrier MA-400-010 was obtained. The specific surface area is shown in Table 1.
[0064] The wide-angle XRD spectrum (10-70°) of MA-400-010 is as follows image 3 As shown in the middle c curve, there are more obvious crystal diffraction peaks, indicating that the amorphous alumina has transformed into γ-Al 2 o 3 crystal phase structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com