Application of thiourea molecule in preparation of antitumor drugs
A technology of antineoplastic drugs and thioureas, which is applied in the direction of antineoplastic drugs, drug combinations, medical preparations containing active ingredients, etc., can solve the problems of toxic and side effects, achieve less toxic and side effects, convenient operation, and good thermal stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
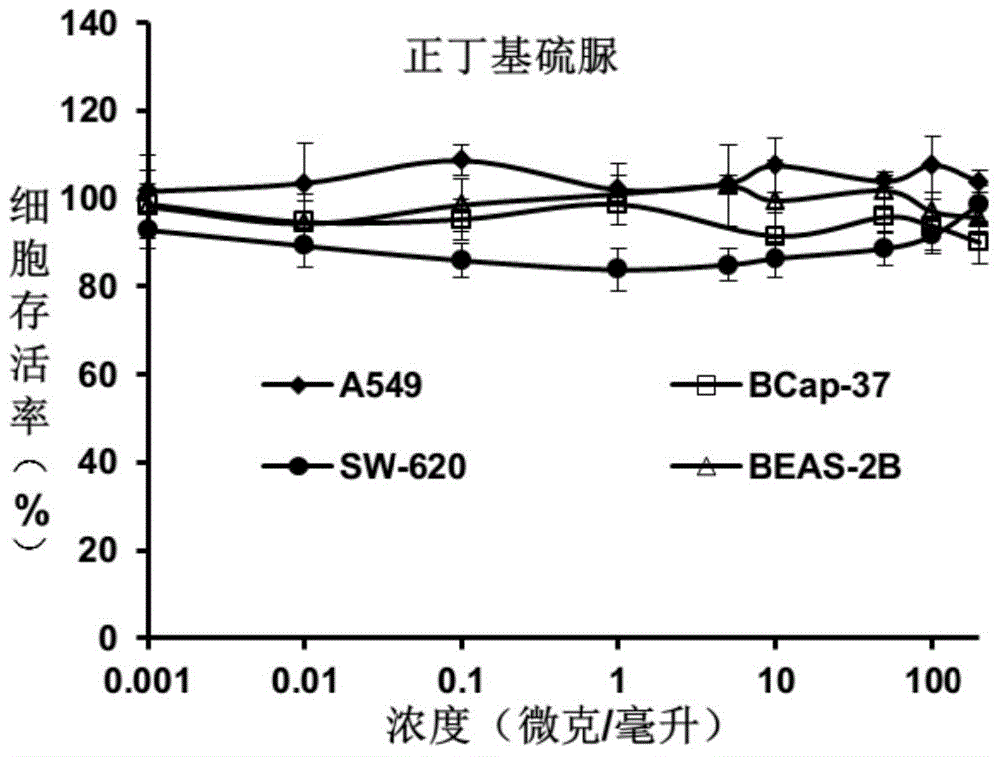
[0034] Embodiment 1 n-butylthiourea
[0035]
[0036] Add n-butylamine (0.735 mL, 7.4 mmol) into 10 mL of dichloromethane, slowly add benzoyl isothiocyanate (0.9 mL, 6.7 mmol) dropwise, and stir overnight at room temperature. After concentration, 10 mL of methanol was added, followed by 3 mL of 5M NaOH in methanol. After stirring for 3 hours, concentrate and pass through the column to obtain colorless crystals of n-butylthiourea with a yield of 81%. 1 H-NMR (400MHz, CDCl 3 ) (mixture of isomers): δ=0.94(t,3H), 1.36-1.44(m,2H), 1.56-1.64(m,2H), 3.20(brs,2H), 6.01(brs,2H), 6.58 (brs,1H).ESI-TOF-MSm / z:[M-H] - , The theoretical value is 131.22, and the actual value is 131.3.
Embodiment 2
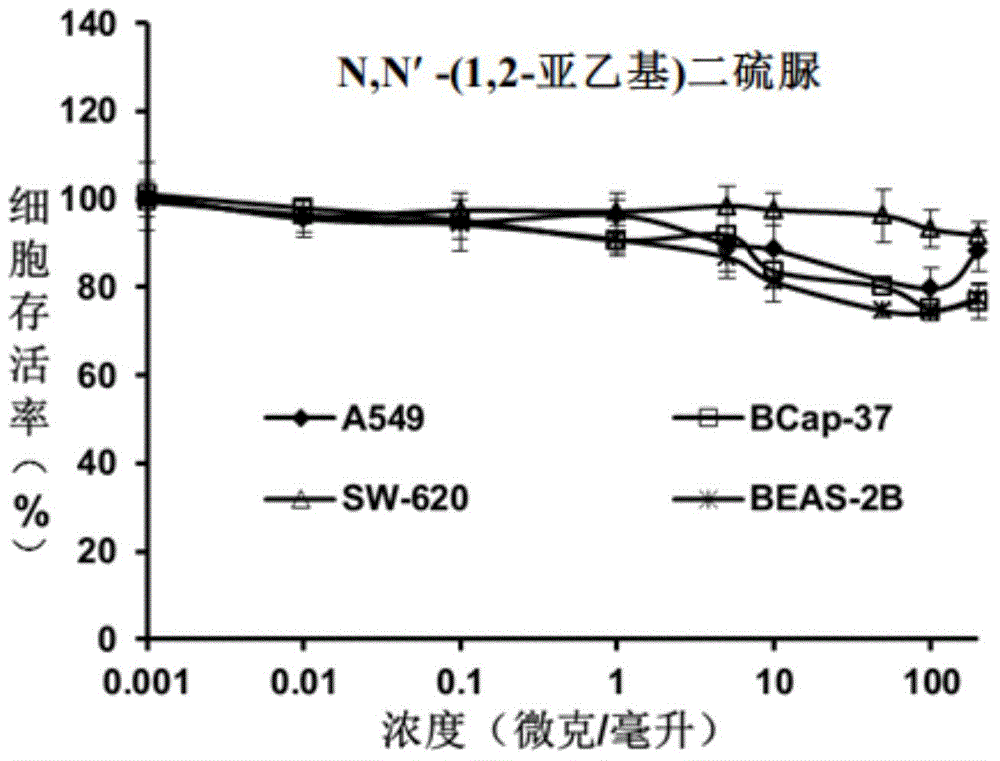
[0037] Embodiment 2N, N'-(1,2-ethylene) dithiourea
[0038]
[0039] Ethylenediamine (0.27g, 4.5mmol) was added into 10mL of dichloromethane, benzoyl isothiocyanate (1.42mL, 10.6mmol) was slowly added dropwise, and stirred overnight at room temperature. After filtering and washing with n-hexane, a light yellow solid was obtained. To this pale yellow solid (1.04 g, 2.68 mmol), 10 mL of methanol was added, and then 3 mL of 5M methanolic NaOH solution was added. After stirring for 3 hours, it was filtered and washed with ice methanol to obtain white solid 1,2-ethyldithiourea with a yield of 85%. 1 H-NMR (400MHz, DMSO-d6) (mixture of isomers): δ=3.12(s,1.46H),3.47(s,2.54H),7.02-7.69(br,6H).ESI-TOF-MSm / z:[M+H] + , The theoretical value is 179.03, and the actual value is 178.9.
Embodiment 3
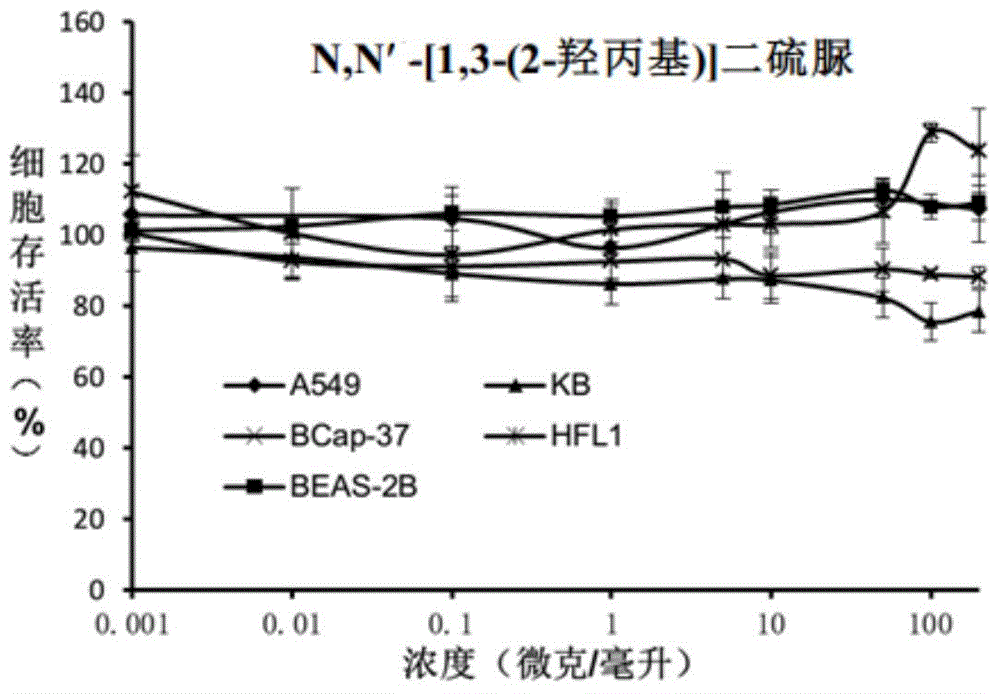
[0040] Embodiment 3N, N '-[1,3-(2-hydroxypropyl)] dithiourea
[0041]
[0042] 1,3-Diamino-2-propanol (0.37g, 4.1mmol) was added to 10mL of dichloromethane, slowly added dropwise to benzoyl isothiocyanate (1.58g, 9.7mmol), stirred at room temperature After 2 hours, filtration gave a pale yellow solid. The light yellow solid was added to 10 mL of methanol, and then 3 mL of 5M methanolic NaOH solution was added, and stirred overnight at room temperature. After concentration, the pure product 1,3-(2-hydroxypropyl)dithiourea was obtained by passing through the column with a yield of 70%. 1 H-NMR (400MHz,D 2 O) (mixture of isomers): δ=3.19(s,1H), 3.28(s,1H), 3.48(s,1H), 3.61(s,1H), 3.99(m,1H). 1 H-NMR (400MHz, DMSO-d6) (mixture of isomers): δ=2.99-3.99(br,1H),3.46(brs,2H),3.72(s,1H),5.15(br,1H),7.06 (brs,4H),7.44-7.55(br,2H).ESI-TOF-MSm / z:[M+H] + , The theoretical value is 209.04, and the actual value is 209.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com