Method for synthesizing diselenides
A technology of diselenide and selenium powder, which is applied in the direction of organic chemistry, can solve the problems of difficult large-scale production, polluting the environment, and long routes, and achieves the effects of low equipment corrosion, large load capacity, and less solid waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 180mg (0.75mol) sodium sulfide nonahydrate and 237mg (3mol) selenium powder into the high-pressure sealed tube, vacuumize and fill with nitrogen, take 2mL of water into the high-pressure sealed tube, fill with nitrogen, place in an oil bath at 140°C and stir for 48 hours .
[0017] Weigh 274 mg (2 mol) of n-bromobutane and 1 mL of ethanol into a high-pressure sealed tube, purging with air, and stirring in an oil bath at 100°C for 48 hours. After the reaction was completed, it was extracted with ether, and the product was separated by climbing a board, and the yield was 64%.
Embodiment 2
[0019] Based on the reaction reported in Example 1, the effects of each reaction at different temperatures in the two steps were investigated, and the experimental results are shown in Tables 1 and 2.
[0020] Table 1: The temperature of step 2 is unchanged, only the temperature of step 1 is changed:
[0021]
[0022] In the above table rt means room temperature.
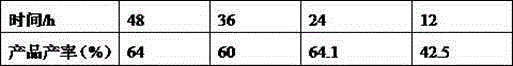
[0023] Table 2: The temperature of step 1 is unchanged (140°C), only the temperature of step 2 is changed:
[0024]
[0025] In the above table rt means room temperature.
[0026] It can be seen from the above table that when the reaction temperature of step 1 is 140°C and the reaction temperature of step 2 is 100°C, the yield is the highest.
Embodiment 3
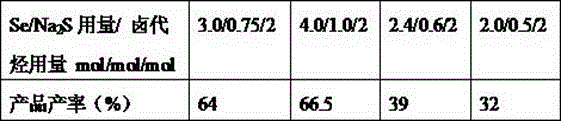
[0028] Other conditions are the same as in Example 1, and the influence of different reactant dosages on the reaction is checked, and the experimental results are shown in Table 3.
[0029] The impact of table 3 reactant dosage
[0030]
[0031] As can be seen from the above table, the yield is the highest when the selenium reagent is 1.5 equivalents (Example 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com