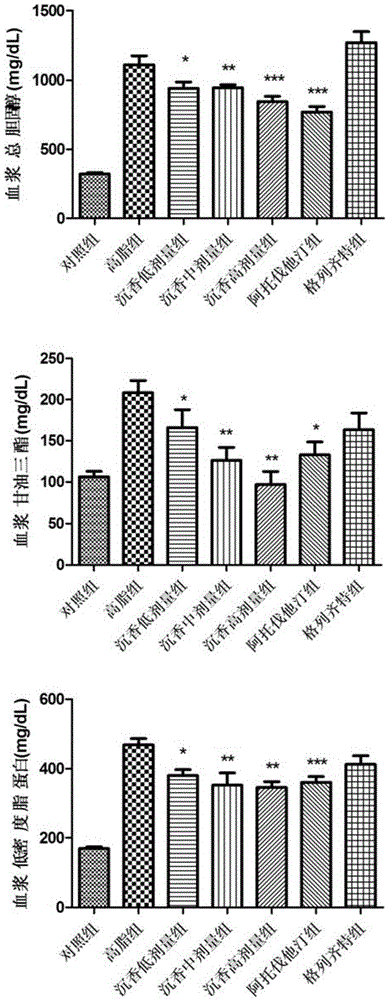
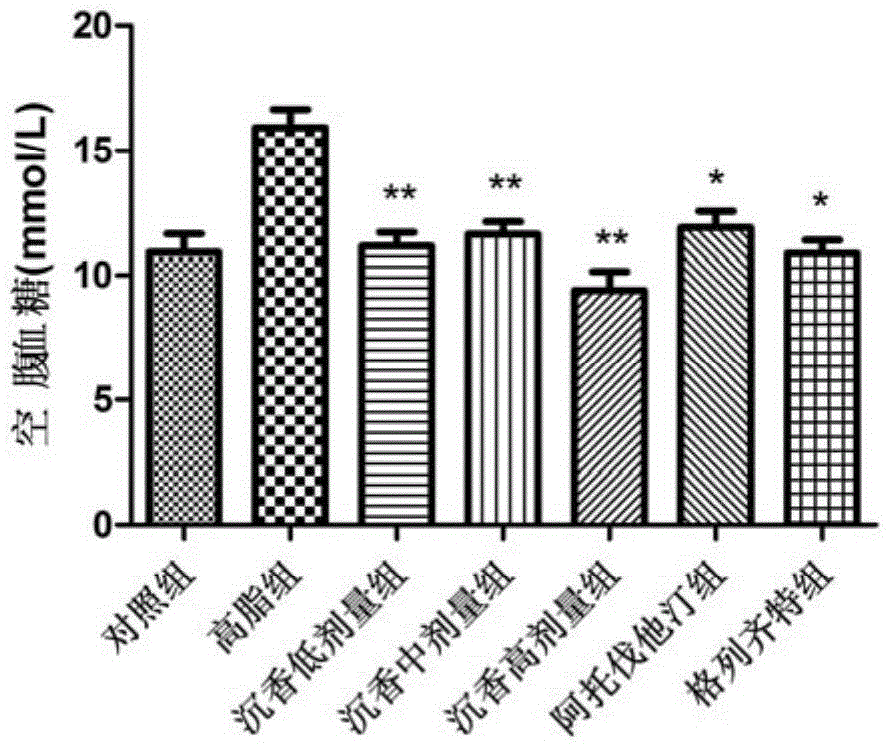
Lignum aquilariae resinatum extract, pharmaceutical composition with same and application thereof
A technology of agarwood extract and composition, which is applied in the field of preparation of medicines for diseases caused by disorders of glucose and lipid metabolism, and can solve the literature reports that no agarwood extract and no effect of agarwood extract on improving glucose and lipid metabolism disorders, There are no problems such as the insulin-stimulating effect of the agarwood extract, and the effect of improving the injection glucose tolerance, improving the impaired glucose tolerance, and improving angina pectoris is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Preparation of Agarwood extract of the present invention
[0051] (1) After pulverizing 500 g of agarwood, reflux extraction with 4 L of 95% by volume ethanol aqueous solution for 2 hours to obtain an extract; repeat the extraction twice in the same way, and combine the extracts.
[0052] (2) Concentrate and dry the extract obtained in step (1) under reduced pressure to obtain 93g of extract; dissolve the extract with 500ml of water, extract 3 times with petroleum ether (1000ml / time), and combine the extracts 3 times to obtain petroleum ether extract; and then extract the aqueous layer with ethyl acetate 3 times (1000ml / time), and combine the three extracts to obtain the ethyl acetate extract.
[0053] (3) The ethyl acetate extract obtained in step (2) was concentrated and dried under reduced pressure to obtain 46 g of ethyl acetate extract.
Embodiment 2
[0054] Example 2: Experimental Research on Insulin Secretion Stimulation of Agarwood Extract of the Present Invention
[0055] 1. Experimental materials:
[0056] 1. Drugs: (1) Test drug: Agarwood extract of the present invention; (2) Positive control drug: Atorvastatin (Pfizer Pharmaceutical Co., Ltd., H20051407), Gliclazide (Tianjin Huajin Pharmaceutical Co., Ltd. , H10910053).
[0057] 2. Animals: SPF grade C57BL / 6ApoE- / - mice, 6-8 weeks old, weighing 19-23g, male; provided by the Animal Center of Peking University Health Science Center.
[0058] 3. Reagents and instruments: Glucose (Beijing Baierdi Biotechnology Co., Ltd.); Insulin injection (NovoNordisk, Denmark); Mouse insulin ELISA quantitative detection kit (Mercodia, Sweden); blood glucose meter and blood glucose test paper (Roche Vitality, GU type, Germany); multi-functional microplate reader (TECAN, Switzerland, M1000 type); centrifuge (Eppendorf, USA, 5424R type); electronic balance (Switzerland METTLERTOLEDO ...
Embodiment 3
[0090] Example 3: Experimental Research on Insulin Secretion Stimulation of Agarwood Extract of the Present Invention
[0091] 1. Experimental materials:
[0092] 1. Cells: Hamster HIT-T15 cells
[0093] 2. Test drug: Agarwood extract of the present invention
[0094] 3. Reagents and instruments: KRB buffer (Krebs-Ringer Bicarbonate Buffer); RPMI1640 cell culture medium and fetal bovine serum (Corning, USA); Gliclazide (China Institute for Food and Drug Control); cell culture box (SANYO, MCO -18AIC); ultra-clean bench (ESCO company, OptiMair); centrifuge (Eppendorf, 5424R type); multifunctional microplate reader (Switzerland TECAN company, M1000 type); mouse insulin ELISA quantitative detection kit (Sweden Mercodia company ).
[0095] 2. Experimental method:
[0096] HIT-T15 cells were cultured with RPMI1640 complete medium containing 10% (volume percentage) fetal bovine serum, and when they grew logarithmically, they were digested with 0.25% trypsin, and then resuspende...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com