Method for preparing zeolite ssz-35
A technology of SSZ-35 and zeolite, applied in the field of preparing zeolite SSZ-35, can solve the problems of expensive, complicated SSZ-35, complicated SDA and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Synthesis of N,N-diethyl-2,3-dimethylpiperidinium cation
[0083] A 3-neck round bottom flask (equipped with mechanical stirrer, heating mantle, and reflux condenser) was charged with one molar equivalent of methanol in methanol sufficient to prepare a 0.5M solution for 2,3-dimethylpiperidine. 2,3-Dimethylpiperidine (note that 2,3-dimethylpiperidine can be purchased directly from commercial sources or prepared by metal catalyzed hydrogenation of 2,3-lutidine). To this solution was added 1.5 molar equivalents of potassium bicarbonate. The solution was stirred for several minutes, then 2.5 molar equivalents of iodoethane were added dropwise via the addition funnel. Once the iodoethane feed was complete, the reaction mixture was heated at reflux (about 55°C) for several hours. The heat was turned off and the reaction mixture was then stirred at room temperature for a further 48 hours. The progress of the reaction was monitored by NMR. Once complete, the reaction mixtur...
Embodiment 2
[0089] Synthesis of N,N-Dimethyl-2-isopropylpiperidinium Cation
[0090] Starting from 2-isopropylpiperidine in methanol, potassium bicarbonate and methyl iodide as reactants, N,N-dimethyl-2-isopropylpiperidine was synthesized in the manner described in Example 1 Onium cation. The reaction afforded N,N-dimethyl-2-isopropylpiperidinium iodide in greater than 95% yield. The iodide counterion was exchanged with hydroxide ion in the manner described in Example 1 to obtain the corresponding N,N-dimethyl-2-isopropylpiperidinium hydroxide in equivalent yield.
[0091] Scheme 2 below describes the synthesis of SDA.
[0092] Schema 2
[0093]
[0094] N,N-Dimethyl-2-isopropylpiperidinium
Embodiment 3
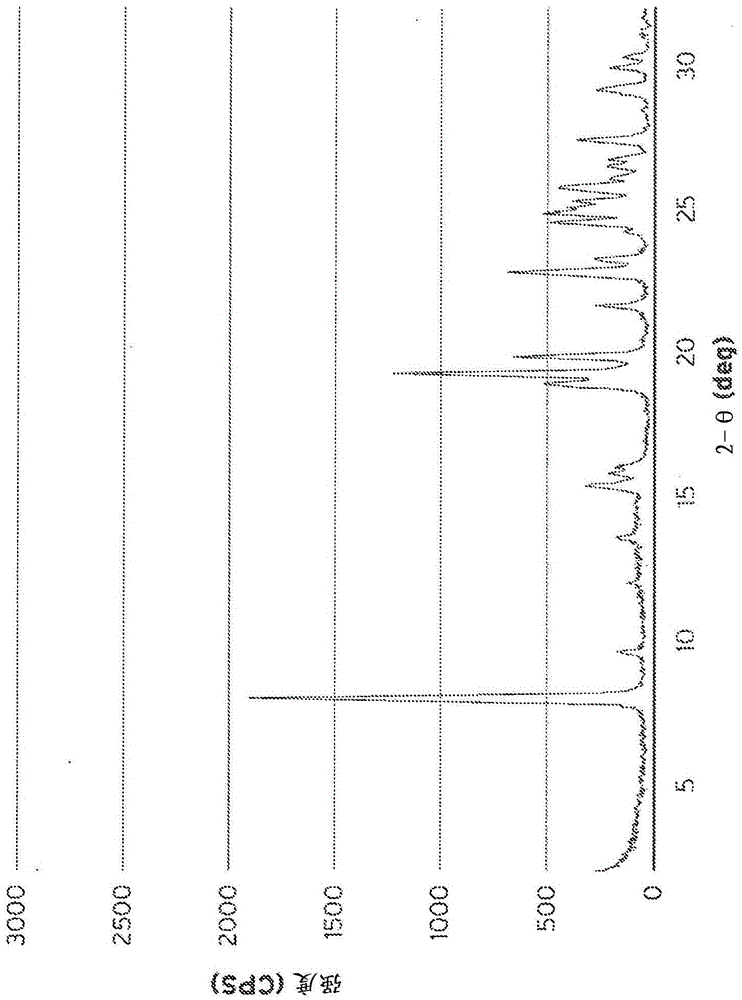
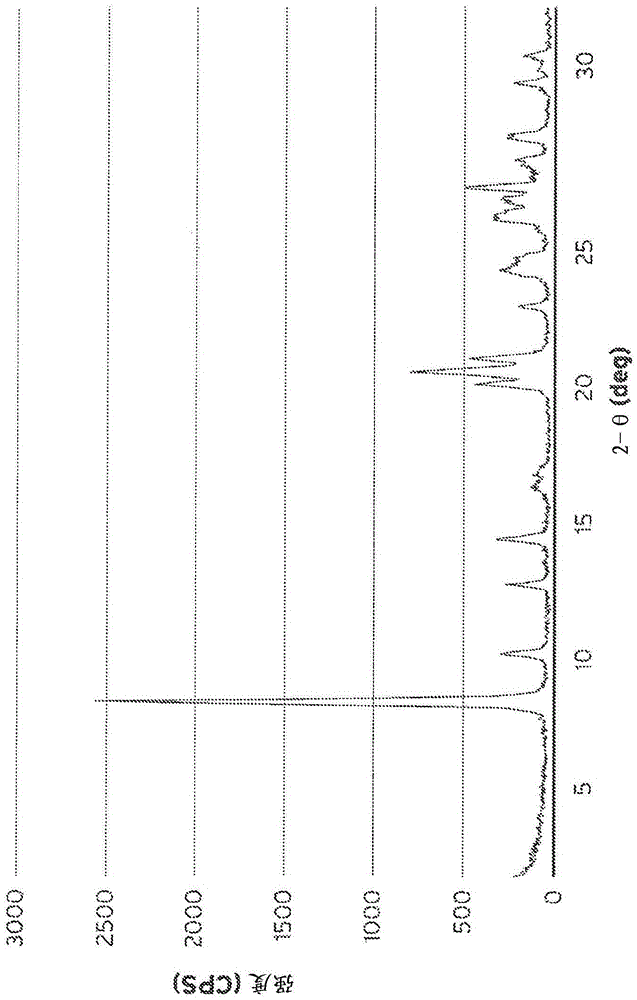
[0096] Synthesis of aluminosilicate SSZ-35 (Al-SSZ-35) using N,N-diethyl-2,3-dimethylpiperidinium cation
[0097] 4.5 grams of a 0.55M solution of N,N-diethyl-2,3-dimethylpiperidinium hydroxide, 1.5 grams of 1N aqueous NaOH, and 4.6 grams of deionized water were all mixed in a 23 mL Teflon liner. To this solution was added 0.035 grams of Reheis F-2000 aluminum hydroxide and stirred until dissolved. Subsequently, 0.9 g of M-5 fumed silica and the solution was stirred until a very homogeneous gel was obtained. The resulting gel was enclosed in a Teflon liner and placed in an autoclave, heated in an oven on a rotating spit at 170°C for 5 days. The gel mixture turned into a clear solution with finely powdered solids settled on the bottom of the Teflon inner substrate. The solid mixture was filtered into a fritted glass funnel. The collected solids were washed thoroughly with 1 liter of deionized water and left to dry under vacuum overnight. The solid was further dried in an ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore volume | aaaaa | aaaaa |
| External area | aaaaa | aaaaa |
| Bet surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com