Preparation method of tilmicosin
A technology of tilmicosin and substances, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of easy doping of catalysts and low purity, and achieve reduced reaction time, high purity, and improved The effect of production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
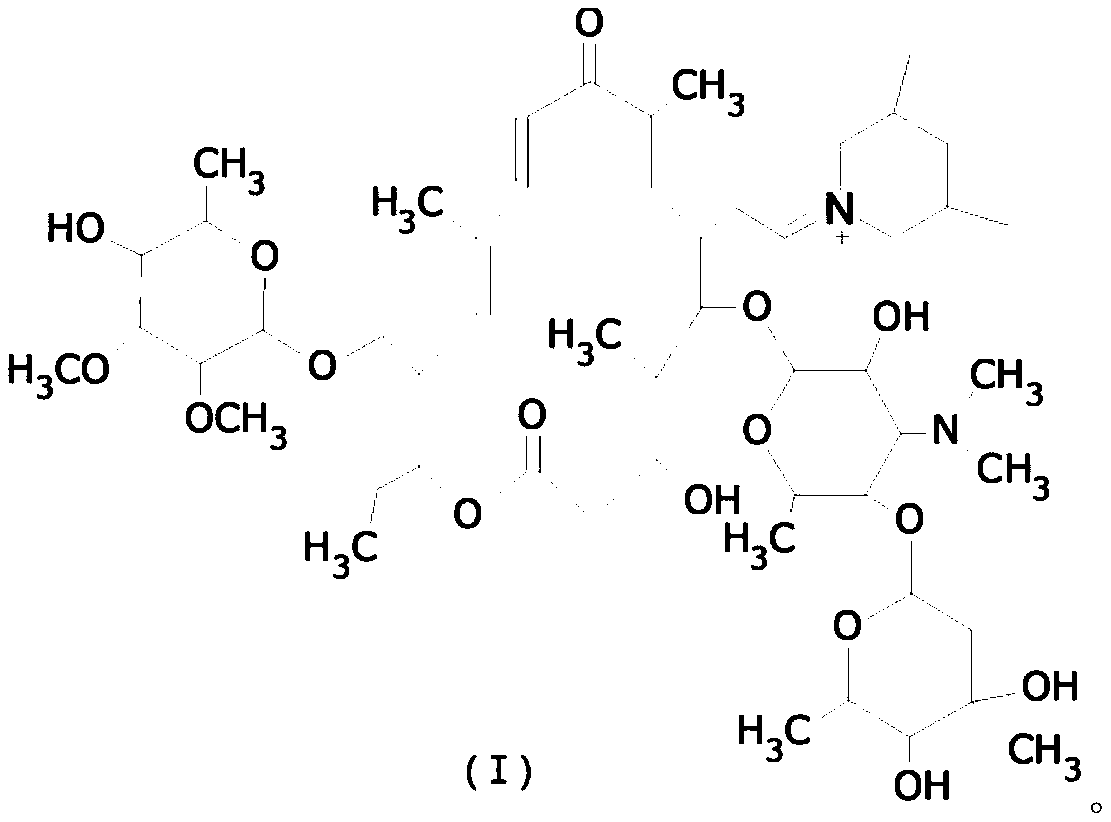
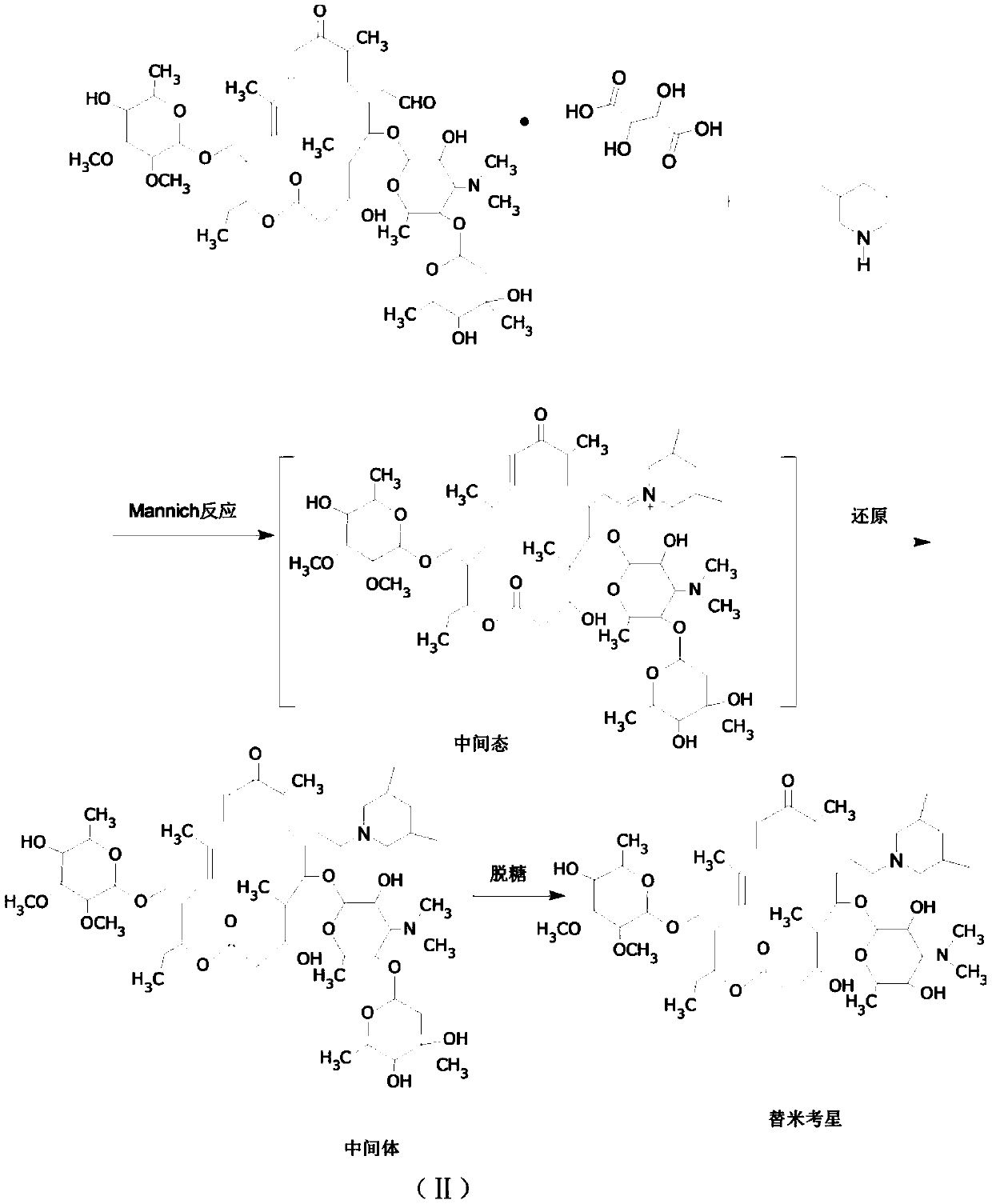
[0028] Add 200g of water to a 500ml flask, add 50g of tylosin tartrate, stir and heat up to 20°C to dissolve, then add liquid caustic soda dropwise, adjust the pH to 8, add 200ml of butyl acetate for extraction, dehydrate the organic layer with 10g of sodium sulfate, add 8g of 3,5-dimethylpiperidine was kept at 50°C for 2 hours, and after that, the temperature was lowered to 30°C and 2.4g of sodium borohydride was added to react at 80°C for 2 hours. After completion, add sulfuric acid aqueous solution dropwise, adjust PH=1, react for 2 hours, complete, separate layers, add sodium hydroxide solution dropwise to the water layer, adjust PH=8, precipitate, filter, wash with water, and dry to obtain tilmicol star. Mass 41g, yield: 82%.
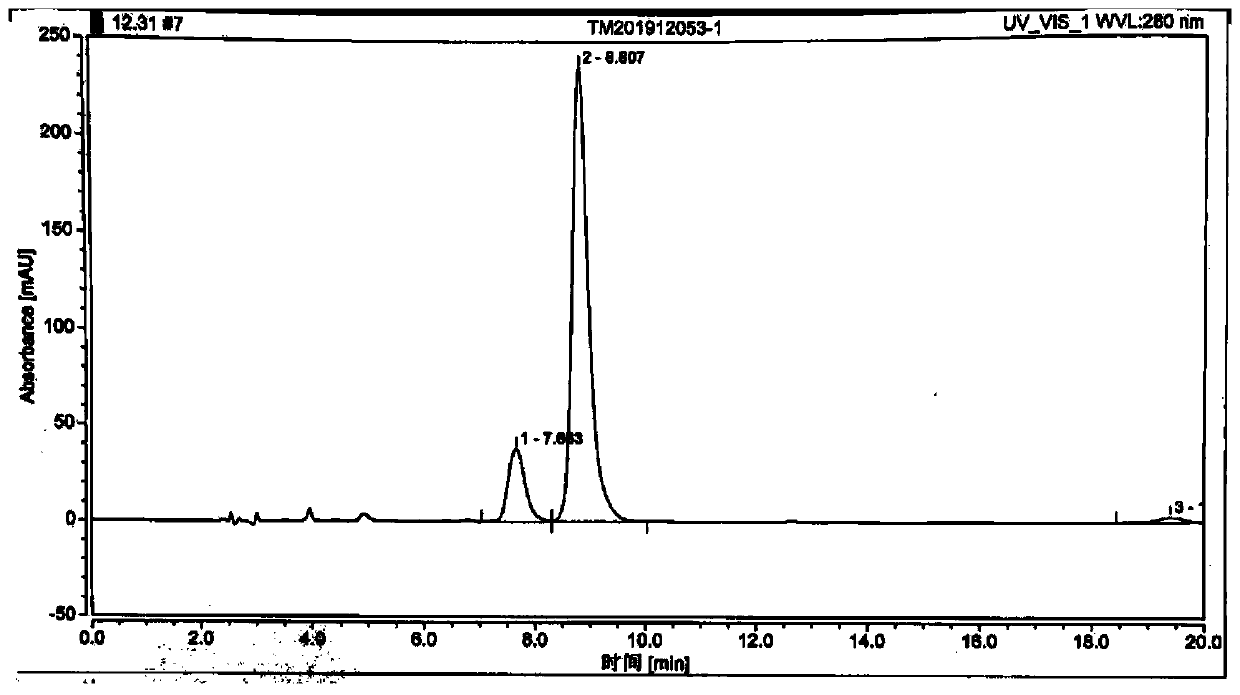
[0029] The prepared tilmicosin was tested for content, and the test conditions were as follows: use octadecylsilane bonded silica gel as filler; use water-acetonitrile-dibutylamine phosphate solution [get 16.8ml of dibutylamine, add phosphoric aci...
Embodiment 2
[0033] Add 500g of water to a 1000ml flask, add 80g of tylosin tartrate, stir and heat up to 40°C to dissolve, then add liquid caustic soda dropwise, adjust the pH to 11, add 350ml of chloroform for extraction, dehydrate the organic layer with 20g of sodium sulfate, add 3, 8.6g of 5-dimethylpiperidine was kept at 40°C for 2 hours. After completion, the temperature was lowered to 10°C and 4.1g of potassium borohydride was added to react for 2 hours. After completion, add sulfuric acid solution dropwise, adjust PH=3, react for 2 hours, complete, separate layers, add sodium hydroxide solution dropwise to the water layer, adjust PH=11, precipitate, filter, wash with water, and dry to obtain tilmicol star. Mass 56.2g, yield: 85%, content 96.3%.
Embodiment 3
[0035] Add 200g of water to a 1000ml flask, add 60g of tylosin tartrate, stir and heat up to 30°C to dissolve, then add liquid caustic soda dropwise, adjust the pH to 10, add 350ml of butyl acetate for extraction, dehydrate the organic layer with 10g of sodium sulfate, add 16.1 g of 3,5-dimethylpiperidine was kept at 65°C for 1.5 hours. After completion, the temperature was lowered to -10°C and 25.1 g of diethylmethoxyborane was added to react for 2 hours. After completion, add sulfuric acid solution dropwise, adjust PH=2, react for 2 hours, complete, separate layers, add sodium hydroxide solution dropwise to the water layer, adjust PH=9, precipitate, filter, wash with water, and dry to obtain tilmicol star. Mass 56.2g, yield: 85%, content 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com