Preparation method of triazole derivatives and application of triazole derivatives as drugs
A triazole, pharmaceutical technology, applied in the field of drug synthesis, can solve the problem of invalidity of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
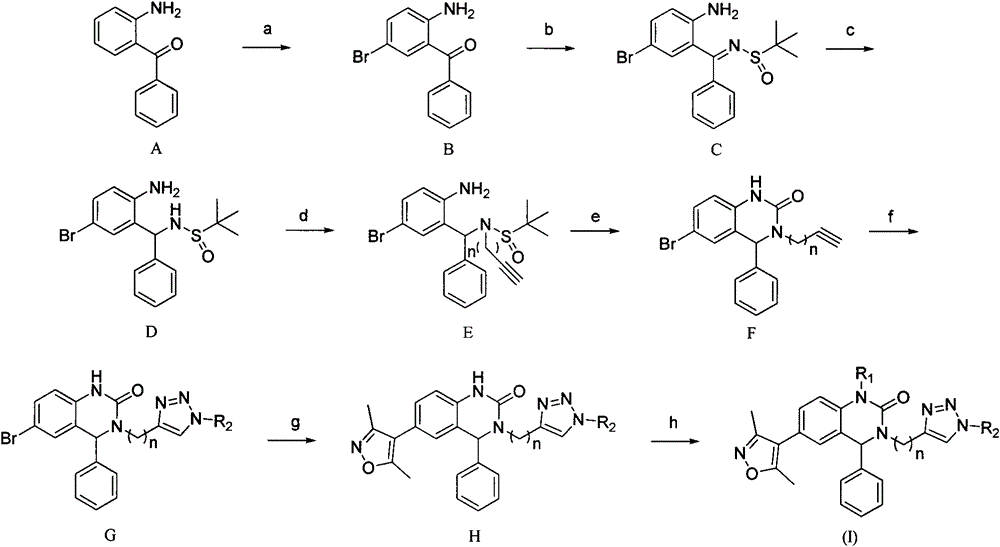
[0066]
[0067] The synthetic route is:
[0068]
[0069] Reagents and conditions: a) N-bromosuccinimide, dichloromethane, -10°C; b) tert-butylsulfinamide, tetraethyl titanate, tetrahydrofuran acetonitrile, 70°C; c) sodium borohydride , tetrahydrofuran, water, room temperature; d) 3-bromopropyne, sodium hydride, N,N-dimethylformamide, 0 ℃; e) triphosgene, tetrahydrofuran, room temperature; f) phenyl azide, sulfuric acid pentahydrate Copper, sodium ascorbate, dichloromethane, methanol, water, room temperature; g) 3,5-dimethylisoxazole-4-boronic acid pinacol ester, [1,1′-bis(diphenylphosphine) di Ferrocene] palladium dichloride dichloromethane complex, potassium carbonate, toluene, methanol, N,N-dimethylformamide, nitrogen, 80°C.
[0070] a) Dissolve 2-aminobenzophenone (5g, 25.35mmol) in 60ml of dichloromethane, stir in a cold trap at -10°C for 10min, and add N-bromosuccinimide (4.74g, 26.62mmol), continued to react in the cold trap for 2 hours, added 30ml of water, ext...
Embodiment 13
[0081]
[0082] The synthetic route is:
[0083]
[0084] Reagents and conditions: a) Iodomethane, sodium hydride, N,N-dimethylformamide, 0°C.
[0085] a) 6-(3,5-dimethylisoxazole)-4-phenyl-3-((1-phenyl-1H-1,2,3-triazole)methyl)-3, 4-Dihydroquinazolin-2(1H)-one (0.12g, 0.25mmol) and methyl iodide (0.071g, 0.5mmol) were dissolved in 2ml of N,N-dimethylformamide and placed in an ice bath at 0°C Stir for 5min, add 60% sodium hydride (0.03g, 0.018mmol) in batches, continue to react in an ice bath for 2 hours, add 10ml of water, solids are precipitated, filter with suction, wash the filter cake with petroleum ether, and dry to obtain 6- (3,5-Dimethylisoxazole)-1-methyl-4-phenyl-3-((1-phenyl-1H-1,2,3-triazole)methyl)-3, 0.11 g of 4-dihydroquinazolin-2(1H)-one is a white solid with a yield of 89.05%. 1 HNMR (300MHz, DMSO) δ8.69(s, 1H), 7.86(d, J=7.9Hz, 2H), 7.58(t, J=7.7Hz, 2H), 7.48(d, J=7.1Hz, 1H) , 7.43-7.23(m, 7H), 7.08(d, J=8.3Hz, 1H), 5.80(s, 1H), 5.26(d, J=15.6Hz, 1H...
Embodiment 14
[0087] The synthetic route is:
[0088]
[0089] Reagents and conditions: a) ethyl azidoacetate, copper sulfate pentahydrate, sodium ascorbate, dichloromethane, methanol, water, room temperature; b) hydrated lithium hydroxide, methanol, water, room temperature; c) tetrahydropyrrole, O -Benzotriazole-tetramethyluronium hexafluorophosphate, diisopropylethylamine, N,N-dimethylformamide, room temperature; d) 3,5-dimethylisoxazole-4 - pinacol borate, [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex, potassium carbonate, toluene, methanol, N,N-dimethylformaldehyde Amide, nitrogen, 80°C.
[0090] a) Mix 6-bromo-4-phenyl-3-propynyl-3,4-dihydroquinazolin-2(1H)-one (0.25g, 0.73mmol) and ethyl azidoacetate (0.14g , 1.1mmol) was dissolved in the mixed solution of 3ml dichloromethane, 3ml methanol and 2ml water, stirred at room temperature, added copper sulfate pentahydrate (0.018g, 0.073mmol), stirred at room temperature for 20min, added sodium ascorbat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com