Pharmaceutical composition and preparation method thereof
A composition and drug technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, block delivery, etc., can solve problems such as low bulk density, inappropriate content measurement values, and reduced selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
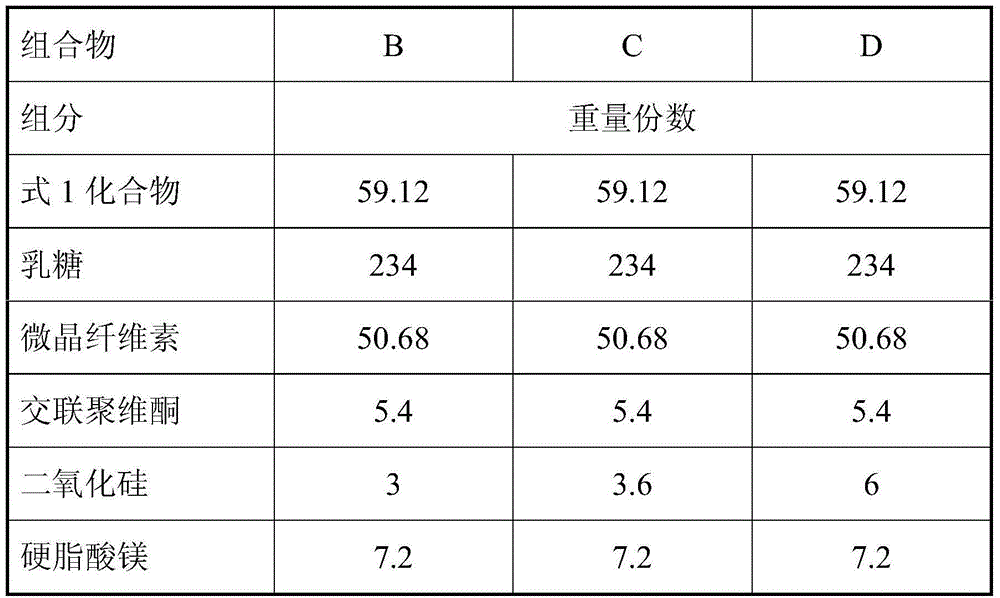
[0035] prescription:
[0036]
[0037] According to the above recipe, take the materials, mix the active substance, lactose, microcrystalline cellulose and crospovidone to obtain an intermediate mixture, then add silicon dioxide and mix with magnesium stearate to obtain the final mixture.
[0038] The static state of each mixture powder in the whole process was directly observed with the naked eye, and it was found that the intermediate mixtures were all electrostatically charged, but the static state of the final mixture of each recipe was different, and the measured particle content was different. The results are shown in Table 1 below.
[0039] Table 1 Example 1 Experiment related results
[0040] combination
A
B
C
D
E
traits
good
good
good
particle content
93.2%
98.7%
99.8%
98.1%
94.9%
[0041] Note: The particle content in this article refer...
Embodiment 2
[0044] Taking the composition C of Example 1 as the object, the influence of different mixing processes on the composition and the final tablet (directly obtained by pressing the composition C) was explored. The process scheme is summarized as follows:
[0045]
[0046] Note: Premix I, premix II, and total mixing are steps performed in sequence, that is, all materials are added to the mixture obtained in the previous step, and then mixed.
[0047] Results: It was observed that the compositions obtained after the total mixing of Schemes 1 and 2 had no static electricity, but the intermediate mixture obtained by the premixing scheme 1 had static electricity, while the intermediate mixtures obtained by the premixing I and the premixing II scheme 2 had no static electricity. Electrostatic; take 6 samples of the mixture obtained in each step, measure its particle content and calculate its deviation RSD, the results are shown in Table 2.1, 2.2.
[0048] Table 2.1 Mixing uniformit...
Embodiment 3
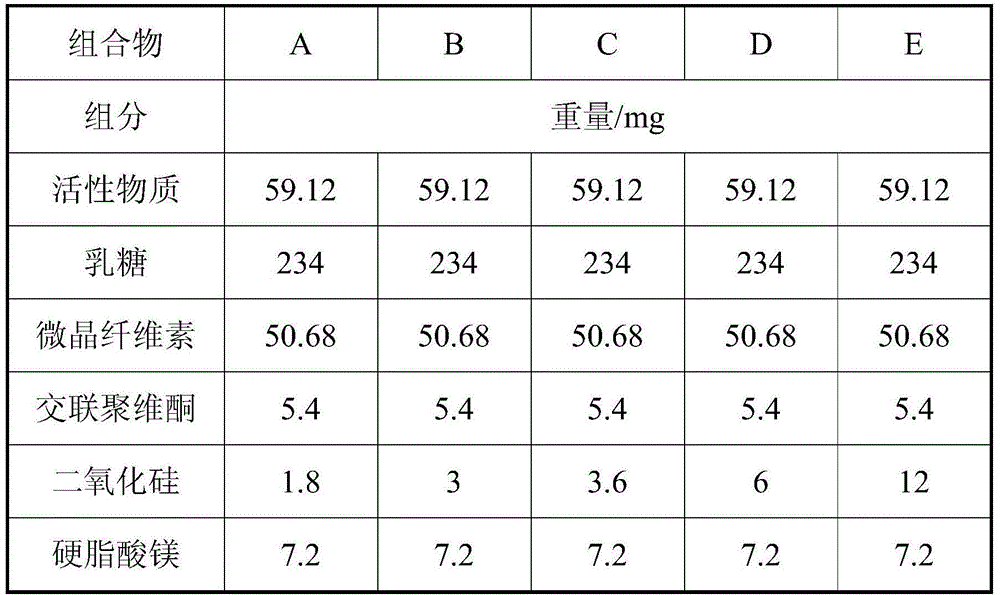
[0067] 1. Prescription
[0068] tablet
C
Element
mg / tablet
mg / tablet
active substance
59.12
59.12
247.72
234
36.96
50.68
Crospovidone
7.2
5.4
silica
1.8
3.6
7.2
7.2
[0069] 2. Preparation process
[0070] 2.1 The samples of the reference preparation were prepared by dry granulation process;
[0071] 2.2 Tablet C uses the preparation process of Scheme 2 in Example 2 to prepare samples, and the hardness is controlled at 5-7kg / cm 2 .
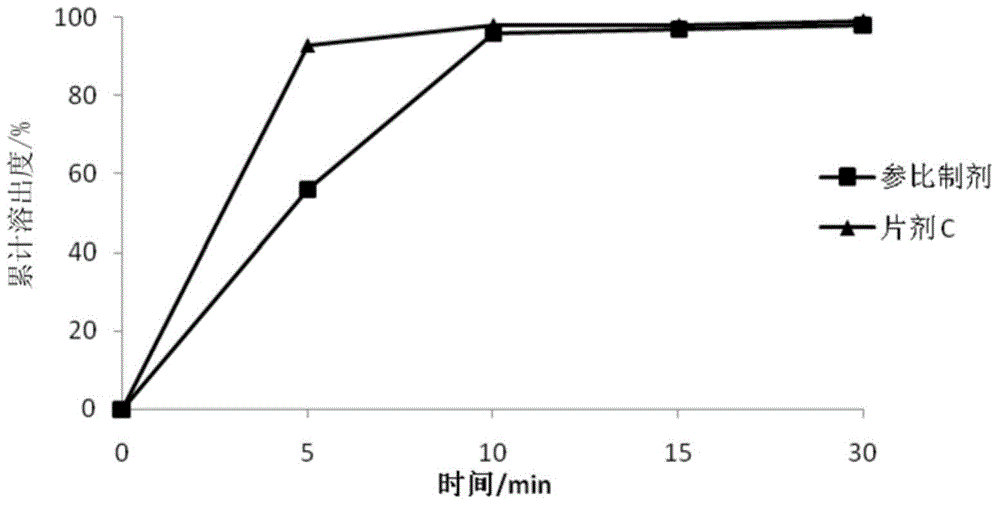
[0072] 3. Dissolution
[0073] The dissolution rate of the tablet was determined according to the dissolution method used in the above table 2.3, and the results of the dissolution data are as follows:
[0074] time / min
tablet C
5
56%
93%
10
96%
98%
15
97%
9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com