Production of electroplating-grade nickel aminosulfonate or electroplating-grade cobalt aminosulfonate through ion exchange method
An ion exchange method and a technology of nickel sulfamate, applied in the field of electroplating chemistry, can solve the problems such as the inability to realize continuous production, and achieve the effects of high strength, high content and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
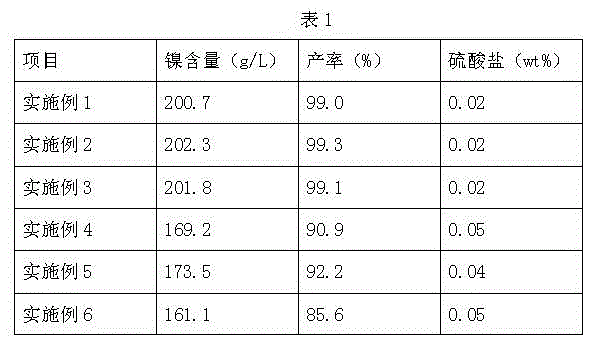
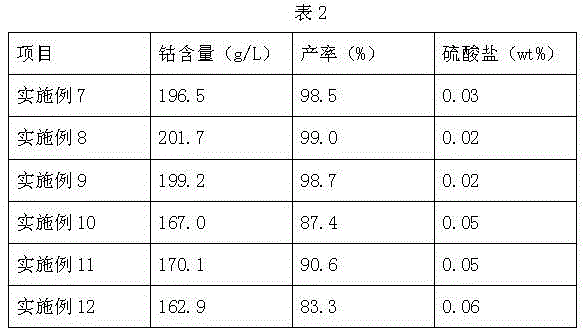
Embodiment 1
[0044] Ion exchange method produces electroplating grade nickel sulfamate, comprises the following steps:
[0045] Step 1: Resin Transformation
[0046] Sodium hydroxide is configured into a solution with a mass concentration of 5%. When the temperature drops below 40 degrees Celsius, the sodium hydroxide solution enters from the upper end of the ion exchange column at a speed of 2 meters per hour, and then the effluent solution is recovered for the following For the first configuration of lye, the amount of resin transformation sodium hydroxide solution is 2 times of resin volume;
[0047] Step Two: Wash
[0048] Use deionized water with a conductivity of less than 10μs / cm to enter from the upper end of the ion exchange column to wash the lye in the ion exchange column, and the washing water is recovered for the preparation of lye;
[0049] Step 3: Ion Exchange
[0050] Pass the nickel salt solution of any concentration through the resin layer that has been transformed at ...
Embodiment 2
[0068] Ion exchange method produces electroplating grade nickel sulfamate, comprises the following steps:
[0069] Step 1: Resin Transformation
[0070] Sodium hydroxide is configured into a solution with a mass concentration of 5%, and when the temperature drops below 40 degrees Celsius, the sodium hydroxide solution enters from the upper end of the ion exchange column at a speed of 4 m / hour and then recovers the effluent solution for the following For the first configuration of lye, the consumption of resin transformation sodium hydroxide solution is 2.5 times of resin volume;
[0071] Step Two: Wash
[0072] Use deionized water with a conductivity of less than 10μs / cm to enter from the upper end of the ion exchange column to wash the lye in the ion exchange column, and the washing water is recovered for the preparation of lye;
[0073] Step Three: Ion Exchange
[0074] Pass the nickel salt solution of any concentration through the transformed resin layer at a flow rate o...
Embodiment 3
[0086] Ion exchange method produces electroplating grade nickel sulfamate, comprises the following steps:
[0087] Step 1: Resin Transformation
[0088] Sodium hydroxide is configured into a solution with a mass concentration of 5%, and when the temperature drops below 40 degrees Celsius, the sodium hydroxide solution enters from the upper end of the ion exchange column at a speed of 5 m / hour and then recovers the effluent solution for the following For the second configuration of lye, the amount of resin transformation sodium hydroxide solution is 3 times of resin volume;
[0089] Step Two: Wash
[0090] Use deionized water with a conductivity of less than 10μs / cm to enter from the upper end of the ion exchange column to wash the lye in the ion exchange column, and the washing water is recovered for the preparation of lye;
[0091] Step Three: Ion Exchange
[0092] Pass the nickel salt solution of any concentration through the resin layer that has been transformed at a flo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com