A kind of sulfated heparin oligosaccharide and its preparation method and application
A technology of sulfated heparin oligosaccharide and heparin oligosaccharide, which is applied in the field of antitumor drugs, can solve the problems of difficult preparation and structure determination of N-acetylated heparin oligosaccharide, no significant improvement in heparanase activity, etc., and achieves good inhibition. Tumor metastatic activity, good heparanase inhibitory activity, small molecular weight effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] The preparation of embodiment 1 heparin oligosaccharide
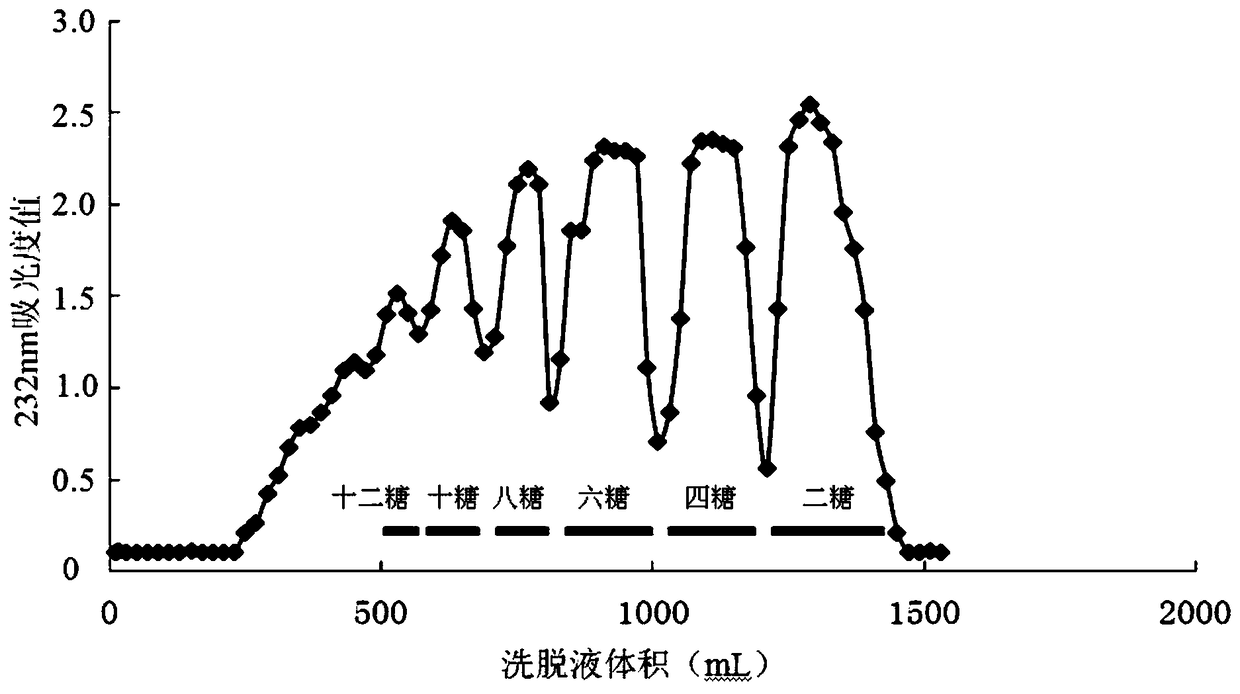
[0092] Take 24g of heparin, add 240mL of Tris-HCl buffer solution, stir to dissolve, add 480IU of heparinase I, stir evenly, and place it at 10°C for enzymolysis reaction for 16h. After the reaction was completed, the reaction mixture was heated to 95° C. for 6 min to inactivate, and ultrafiltered in a 10 KDa ultrafiltration centrifuge tube. The filtrate was separated with a Bio-Gel P-10 (2.5×100cm) chromatographic column, and 0.2M NH 4 HCO 3 As the eluent, collect 1.75-2.15 times the column volume components to obtain the heparin tetrasaccharide mixture, collect 1.35-1.75 times the column volume components to obtain the heparin hexasaccharide mixture, collect 1.05-1.35 times the column volume components to obtain the heparin 8 Sugar-sugar mixture, collect 0.85-1.05 times column volume components to get heparin decasaccharide mixture, collect 0.75-0.85 times column volume components to get heparin dodecaose mix...
Embodiment 2
[0096] Example 2 Preparation of 20%~80% Sulfated Heparin Octaose
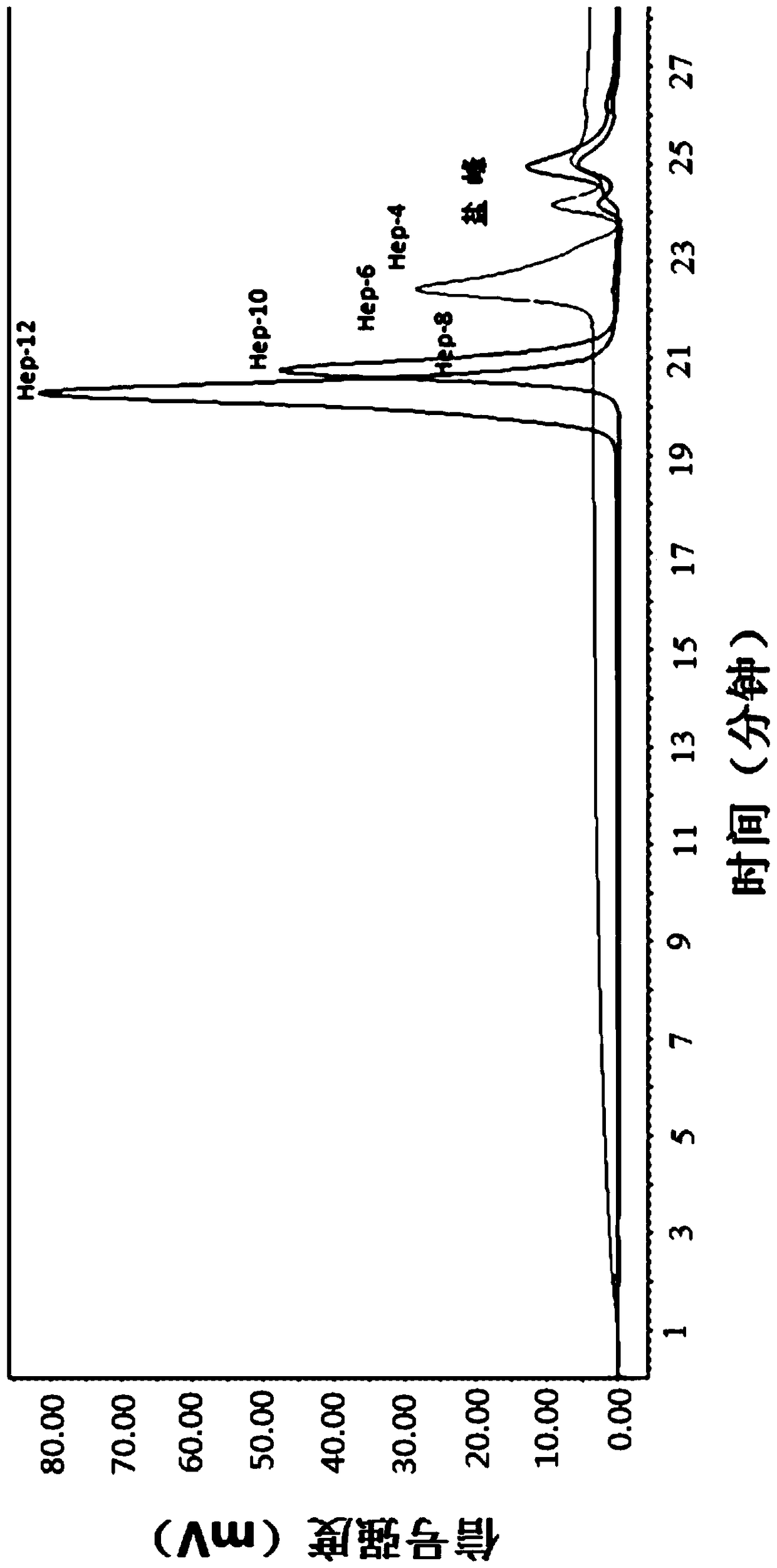
[0097] Weigh 0.54g of heparin octasaccharide raw material, transfer it to a reaction bottle, add 25mL of anhydrous DMF, and stir to dissolve. Weigh 0.86g (CH 3 ) 3 N·SO 3 , gradually added to the above solution under stirring, and stirred for 10 min. The bottle cap was plugged, and the reaction bottle was placed in an oil bath at 80°C and stirred for 4 h. After stopping the reaction, it was naturally cooled to room temperature. The solid was dissolved in 90 mL of pure water, and the pH was adjusted to nearly neutral with 2M NaOH. The solution was transferred to a dialysis bag with a molecular weight cut-off of 100-500 Da, and after three days of dialysis, the solution was washed with BaCl 2 Check for the presence of large amounts of sulfate in the dialysate. If not, first adjust the pH to neutral with 2M NaOH, and concentrate to about 10 mL on a rotary evaporator. Put it on the P2 column, elute with pur...
Embodiment 3
[0106] Example 3 Heparanase Inhibitory Activity of 40% and 60% Sulfated Heparin Octaose
[0107] The method described in the literature [Hammond E, Li CP, Ferro V. Development of a colorimetric assay for heparanase activity suitable for kinetic analysis and inhibitorscreening. Anal. Biochem. 2011; 396: 112-116] was used to determine the concentration of sulfated heparan oligosaccharide Heparanase inhibitory activity, that is, the specific experimental operation is as follows:
[0108]The reaction solution of the experimental group was composed of 40mM sodium acetate buffer solution (pH 5.0), 100mM fondaparinux sodium, and a specific concentration of heparin sulfated octaose, and the reaction solution of the control group was composed of the same Concentration of SST0001 reference substance to replace sulfated heparin octasaccharide. Add 100 μL of the reaction solution of the experimental group or the control group to each well of the 96-well plate, and then add heparanase res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com