Difluoro monomer with photoelectric activity and its use in the preparation of polyarylethersulfone polymers
A technology of high molecular polymer and double fluorine monomer, applied in the direction of organic chemistry, etc., can solve the problems of thermal stability, solubility and film-forming processability of materials, achieve good optoelectronic properties, cheap raw materials, good The effect of thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of polymer P1
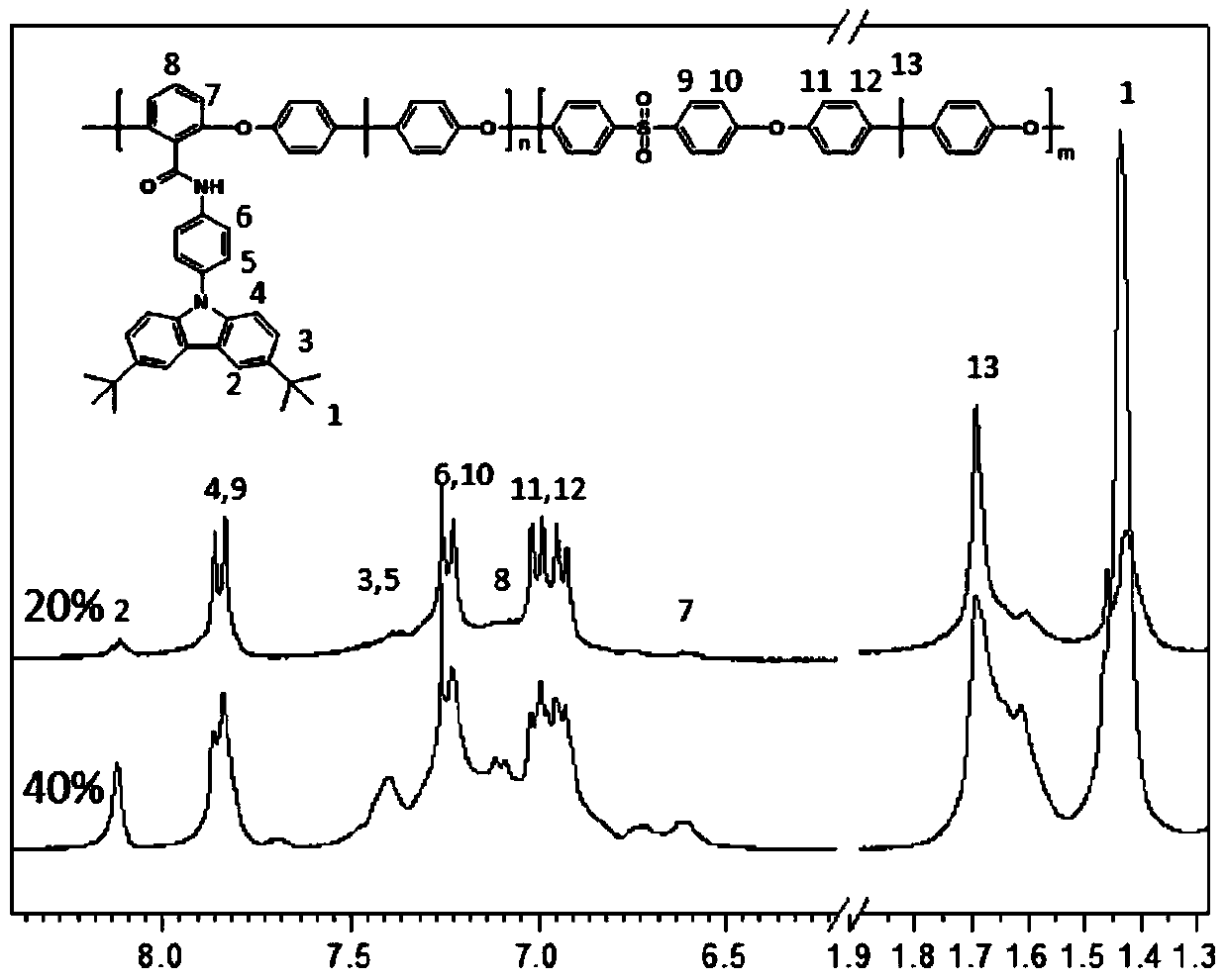
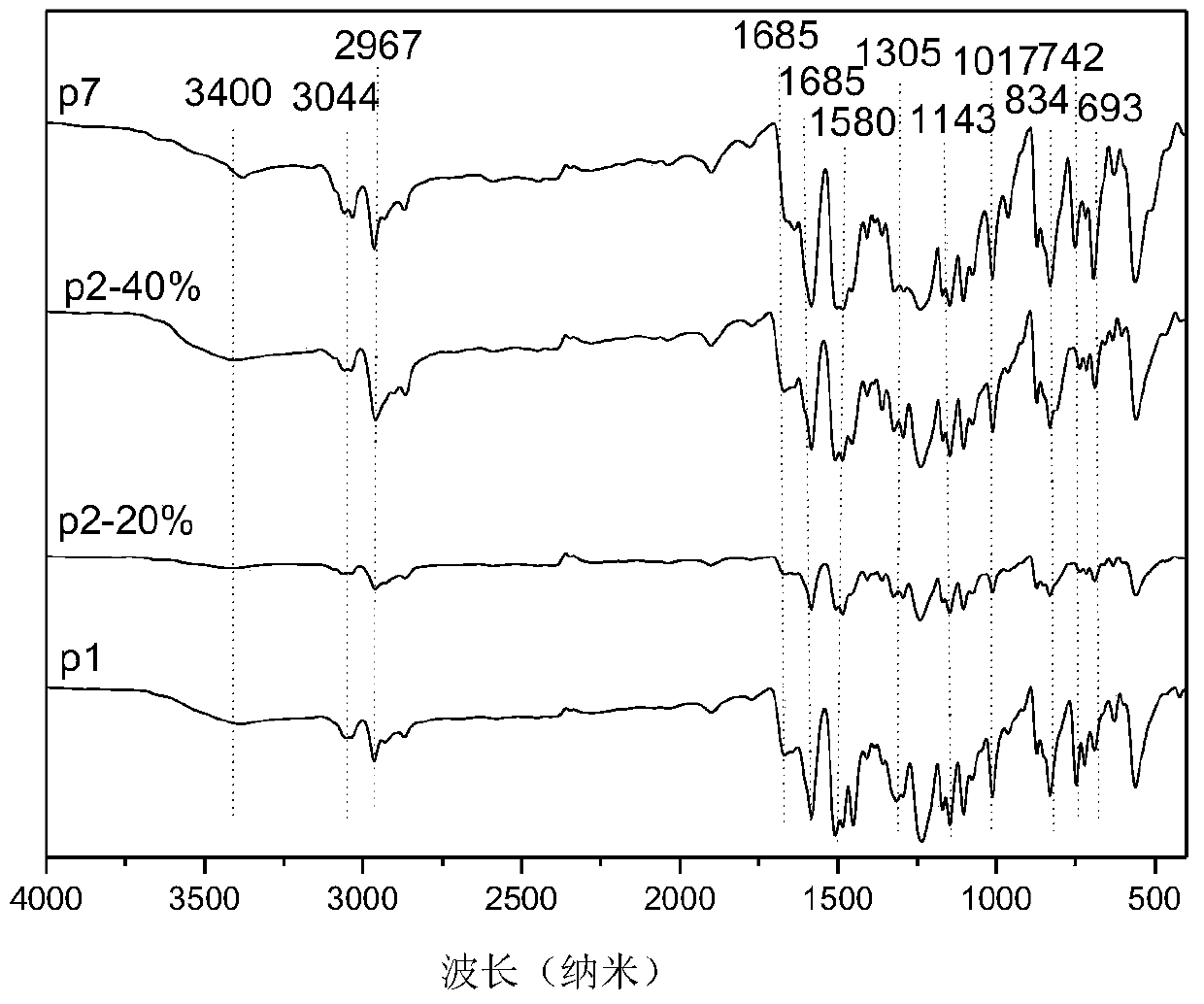
[0042] (1) In a 500 mL two-necked flask, p-aminophenylcarbazole (15.49 g, 60 mmol) was dissolved in 120 mL of dichloromethane, and then 24 mL of triethylamine was added. While stirring under nitrogen atmosphere at room temperature, a solution of 2,6-difluorobenzoyl chloride (11.08 g, 63 mmol) in dichloromethane (120 mL) was slowly added dropwise to the above solution. The reaction was stirred at room temperature for 12h. The solution was washed with water, and the solvent was removed by evaporation. The crude product was recrystallized from a mixture of ethanol and water with a volume ratio of 10:1 to obtain white crystals, which were the product difluoromonomer M1 (20.53 g, 86%). 1H NMR (DMSO-d, rt, 300MHz): δ11.11(s, NH, 1H), 8.26-8.24(d, CH, 2H), 8.02-8.00(d, CH, 2H), 7.65(d, CH ,2H),7.62(t,CH,1H),7.44(d,CH,1H),7.41(d,CH,1H),7.33-7.30(t,CH,2H),7.29-7.26(t,CH, 2H).
[0043] (2) Difluoromonomer M1 (3.984g, 10mmol), bisp...
Embodiment 2
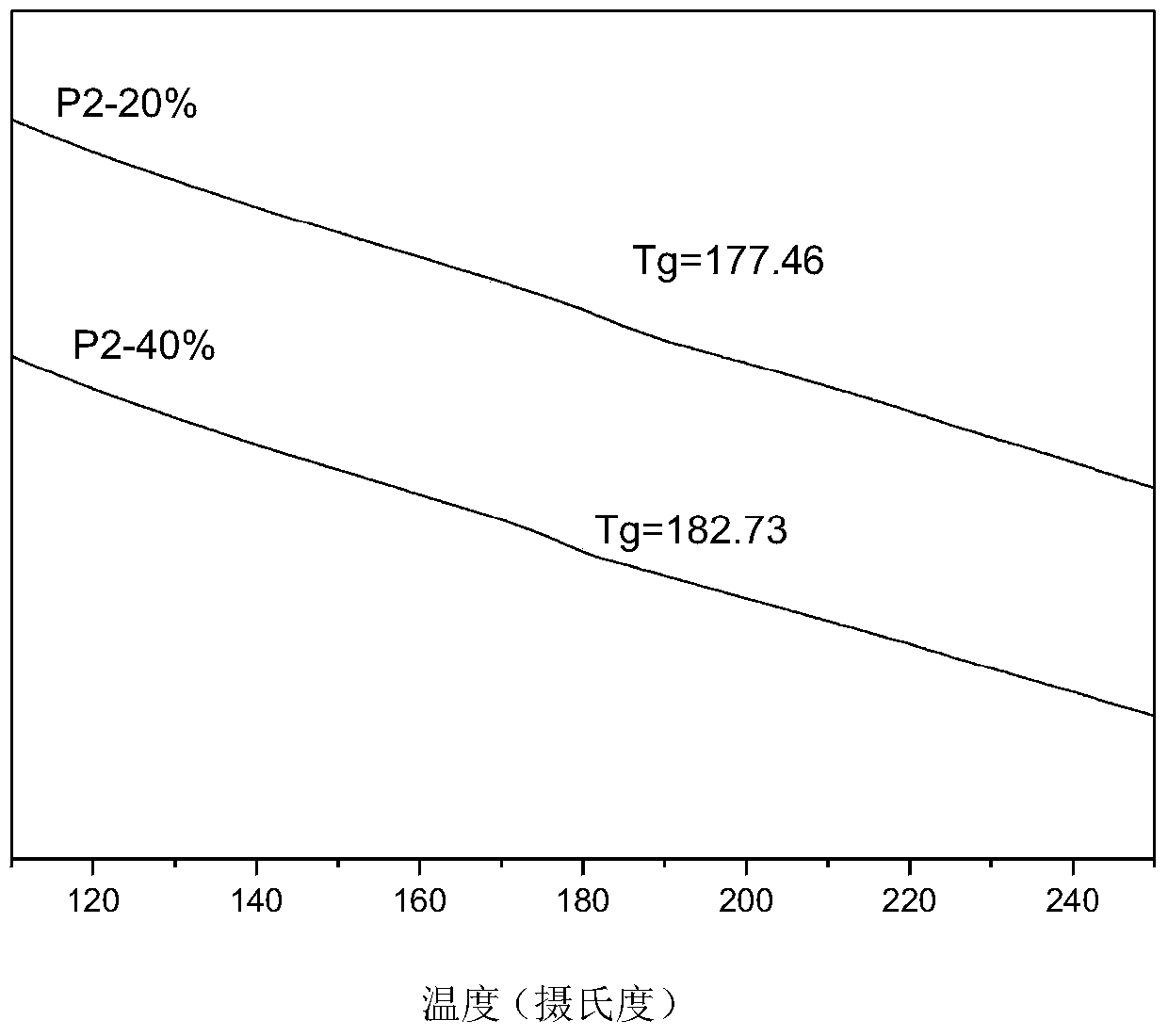
[0045] Embodiment 2: the preparation of polymer P2 series
[0046] (1) Dissolve p-aminophenyl bis-tert-butylcarbazole (18.50 g, 50 mmol) in 100 mL of dichloromethane in a 500 mL two-necked flask, and then add 20 mL of triethylamine. While stirring under nitrogen atmosphere at room temperature, a solution of 2,6-difluorobenzoyl chloride (9.15 g, 52 mmol) in dichloromethane (100 mL) was slowly added dropwise to the above solution. The reaction was stirred at room temperature for 12h. The solution was washed with water, and the solvent was removed by evaporation. The crude product was recrystallized from a mixture of ethanol and water with a volume ratio of 10:1 to obtain white crystals which were the product difluoromonomer M2 (23.62 g, 92%). 1H NMR(DMSO-d,rt,300MHz):δ11.07(s,NH,1H),8.29(d,CH,2H),7.98-7.95(d,CH,2H),7.66(d,CH,2H ),7.62(t,CH,1H),7.59(d,CH,1H),7.49(d,CH,1H),7.33-7.30(t,CH,2H),7.29-7.26(t,CH,2H) , 1.42 (s, CH, 9H).
[0047] (2) Put bisfluoromonomer M2, bisphenol...
Embodiment 3
[0050] Embodiment 3: the preparation method of polymer P7
[0051] (1) In a 500 mL two-necked flask, p-aminotriphenylamine (13.02 g, 50 mmol) was dissolved in 100 mL of dichloromethane, and then 20 mL of triethylamine was added. While stirring under nitrogen atmosphere at room temperature, a solution of 2,6-difluorobenzoyl chloride (9.15 g, 52 mmol) in dichloromethane (100 mL) was slowly added dropwise to the above solution. The reaction was stirred at room temperature for 12h. The solution was washed with water, and the solvent was removed by evaporation. The crude product was recrystallized from a mixture of ethanol and water with a volume ratio of 10:1 to obtain light blue crystals which were the product difluoromonomer M7 (18.03 g, 90%). 1H NMR (DMSO-d, rt, 300MHz): δ10.79(s, NH, 1H), 7.65(d, CH, 1H), 7.62(d, CH, 2H), 7.58-7.56(m, CH, 4H ), 7.31-7.22 (m, CH, 6H), 7.05-7.00 (m, CH, 4H).
[0052] (2) Difluoromonomer M7 (3.20g, 8mmol), bisphenol A (4.56g, 20mmol), 4,4'-dic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com