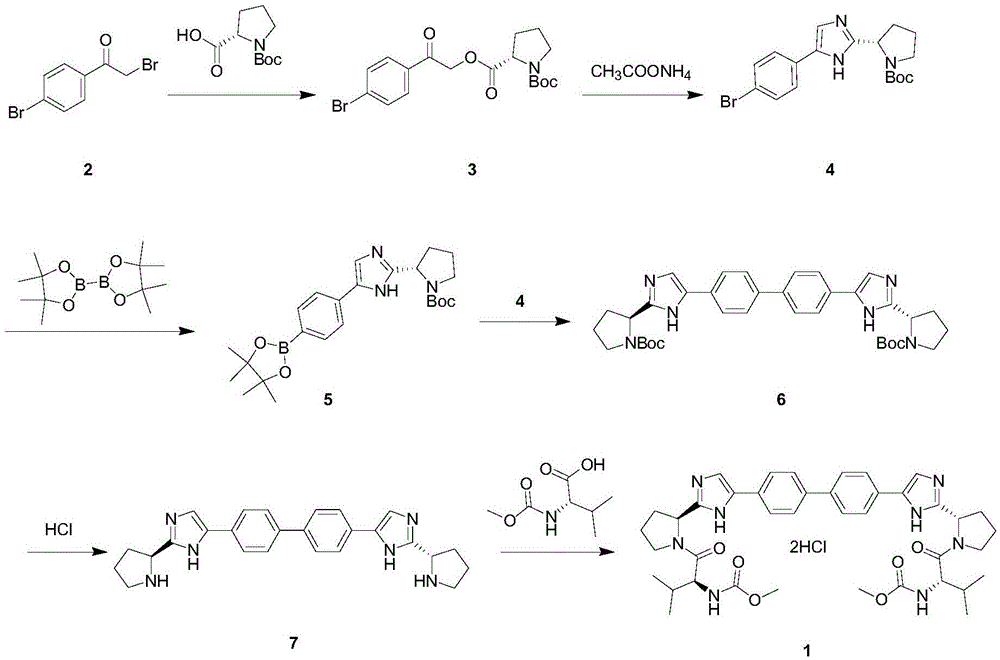
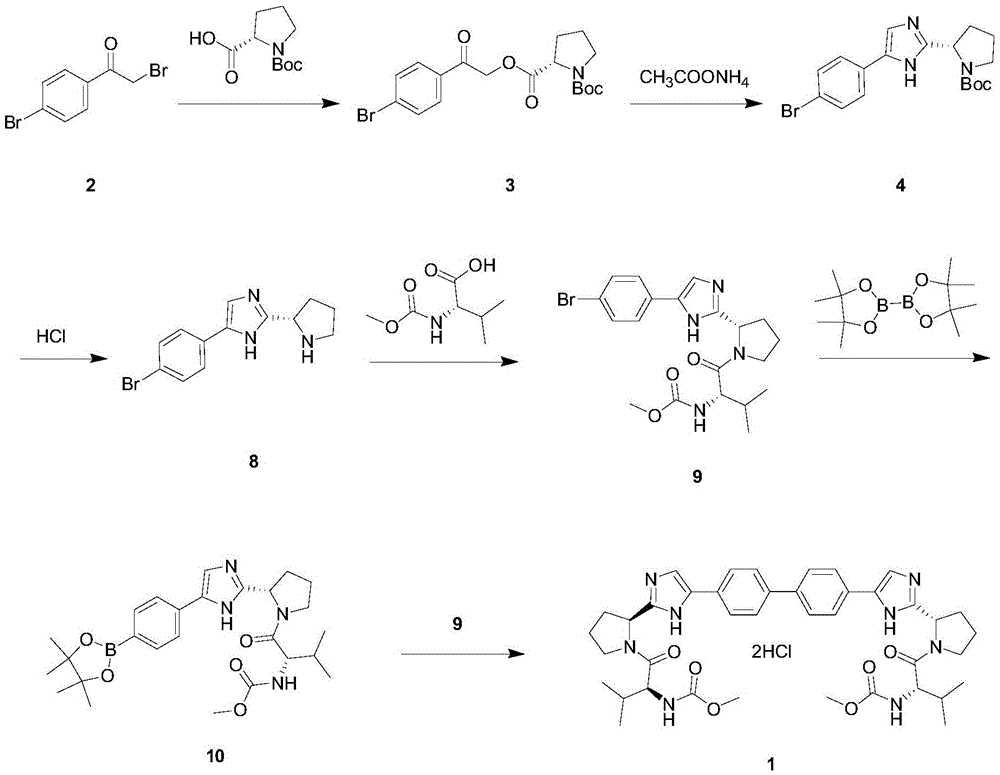
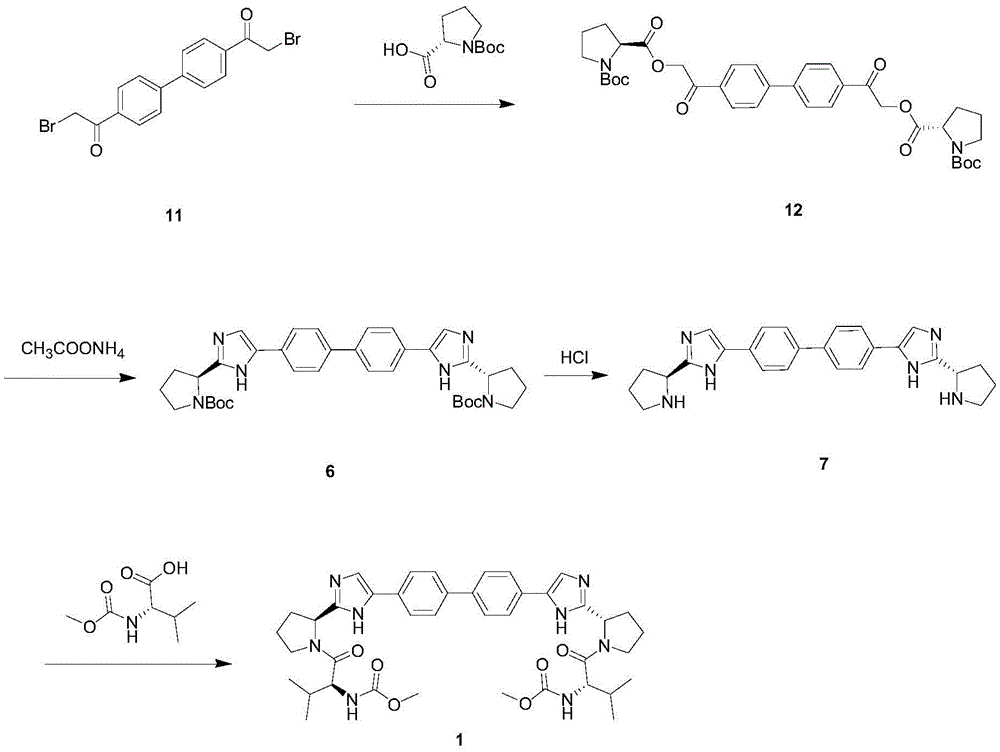
Novel method for synthesizing anti-hepatitis C virus novel medicine daclatasvir
A synthetic method, daclatasvir technology, applied in organic chemistry, peptides, etc., can solve the problems of many synthesis steps and high synthesis cost, and achieve the effect of short synthesis cycle, low production cost and few steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 , 4,4'-two (N-(methoxycarbonyl)-L-valyl-L-proline ester acetyl) biphenyl (14) synthesis
[0034] Add 4,4'-bis(2-bromoacetyl)biphenyl (20g, 50mmol), N-(methoxycarbonyl)-L-valyl-L-proline (29g, 105mmol) into a 500mL three-necked flask ) and acetonitrile (200mL), the reaction solution was a suspension, then cooled to 10°C, and the reaction acid-binding agent diisopropylethylamine (DIPEA) (3.7g, 105mmol) (3.7g, 105mmol) was added dropwise under control of the temperature in the range of 10-20°C, and After the addition is completed, the temperature is controlled in the range of 25-30° C. and stirred for 3-5 hours, and the condensation reaction is completed.
[0035] The reaction solution was concentrated to dryness under reduced pressure, extracted with toluene solution (200 mL), and then washed twice with saturated sodium chloride solution (100 mL). The toluene solution was concentrated to obtain an oily foamy solid (37.5 g), yield 96%.
Embodiment 2
[0036] Example 2 , 4,4'-two (N-(methoxycarbonyl)-L-valyl-L-proline ester acetyl) biphenyl (14) synthesis
[0037] Add 4,4'-bis(2-bromoacetyl)biphenyl (20g, 50mmol), N-(methoxycarbonyl)-L-valyl-L-proline (29g, 105mmol) into a 500mL three-necked flask ) and acetonitrile (200mL), the reaction solution was a suspension, then the temperature was raised to 40°C, and the reaction acid-binding agent diisopropylethylamine (DIPEA) (13.7g, 105mmol) (13.7g, 105mmol) was added dropwise under control of the temperature in the range of 40-50°C. After the addition is completed, the temperature is controlled in the range of 40-50° C. and stirred for 1-2 hours, and the condensation reaction is completed.
[0038] The reaction solution was concentrated to dryness under reduced pressure, extracted with toluene solution (200 mL), and then washed twice with saturated sodium chloride solution (100 mL). The toluene solution was concentrated to obtain an oily foamy solid (35.1 g), yield 90%.
Embodiment 3
[0039] Example 3 , 4,4'-two (N-(methoxycarbonyl)-L-valyl-L-proline ester acetyl) biphenyl (14) synthesis
[0040] Add 4,4'-bis(2-bromoacetyl)biphenyl (20g, 50mmol), N-(methoxycarbonyl)-L-valyl-L-proline (29g, 105mmol) into a 500mL three-necked flask ) and acetonitrile (200mL), the reaction solution was a suspension, then cooled to 10°C, and the reaction acid-binding agent diisopropylethylamine DIPEA (13.7g, 105mmol) was added dropwise under control of the temperature in the range of 10-15°C, and the addition was completed , control the temperature in the range of 10-15°C and stir for 8-10 hours, and the condensation reaction is completed.
[0041] The reaction solution was concentrated to dryness under reduced pressure, extracted with toluene solution (200 mL), and then washed twice with saturated sodium chloride solution (100 mL). The toluene solution was concentrated to obtain an oily foamy solid (36.2 g), yield 93%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com