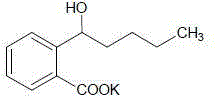
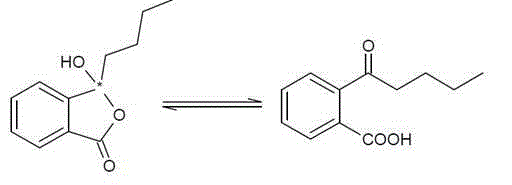
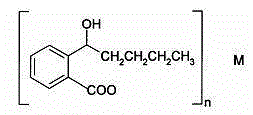
Application of butylphthalide or derivatives thereof in preparing drug for treating or preventing brain paralysis
A technology of butylphthalide and derivatives, applied in the field of pharmaceutical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 Preparation of an animal model of cerebral palsy.
[0077] 1. Animal model preparation:
[0078] 60 clean-grade mature female rats and 30 male rats, weighing 200-280g. Female rats and male rats were reared routinely at room temperature (25°C±5°C), free to eat and drink, with half and half light and dark cycles (12h / 12h), after two weeks of environmental adaptation, at 17:00 p.m. at 2:1 (female: Male) were kept together in the same cage, and the vaginal smear was checked at 8 o'clock in the morning of the next day, and the sperm found was regarded as the 0th day of pregnancy, and the pregnant mice were raised in another cage;
[0079] The pregnant rats were randomly divided into the control group (n=20) and the model group (n=22), and the pregnant rats in the model group on the 17th day of pregnancy were given lipopolysaccharide (serotype 0.55: B5 strain, Sigma company) 450 μg / kg, continuously Two days of intraperitoneal injection, the same dose of normal sa...
Embodiment 2
[0098] Embodiment 2: Butylphthalide pair Big Therapeutic or preventive effects of cerebral palsy in mice.
[0099] 1. Preparation of animals and models:
[0100] 1.1 Animal model preparation:
[0101]120 clean-grade mature female rats and 60 male rats, weighing 200-280g. Female rats and male rats were reared routinely at room temperature (25°C±5°C), free to eat and drink, with half and half light and dark cycles (12h / 12h), after two weeks of environmental adaptation, at 17:00 p.m. at 2:1 (female: Male) were kept together in the same cage, and the vaginal smear was checked at 8 o'clock in the morning of the next day, and the sperm found was regarded as the 0th day of pregnancy, and the pregnant mice were raised in another cage;
[0102] The pregnant rats were randomly divided into the control group (n=15) and the cerebral palsy group (n=90), and the pregnant rats in the model group on the 17th day of pregnancy were given lipopolysaccharide (serotype 0.55: B5 strain, Sigma...
Embodiment 3
[0133] Example 3: Therapeutic or preventive effects of butylphthalide derivatives on cerebral palsy in guinea pigs.
[0134] 1. Preparation of animals and models:
[0135] 1.1 Animal model preparation:
[0136] 100 clean-grade mature female rats and 50 male rats, weighing 200-280g. All the other are with embodiment 2;
[0137] Randomly select 20 pups from the control group and 100 pups from the cerebral palsy group (divided into 5 groups). The control group was fed routinely for 42 days. On the basis of routine feeding, the other groups were given different doses of butylphthalide derivatives, and neurological examination was carried out. Behavioral testing and motor function assessment.
[0138] 1.2 Grouping and administration:
[0139] (1) Normal control group: intragastric administration of the same volume of normal saline;
[0140] (2) Cerebral palsy model group: 20 cerebral palsy animal models were administered with the same amount of normal saline;
[0141] (3) 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com