Copper complexes of amino acids and phenanthroline or phenanthroline derivative and preparation method and application thereof
A technology of o-phenanthroline and copper complexes, which can be used in copper organic compounds, drug combinations, antitumor drugs, etc., can solve the problems of not having too much effect, lack of chemical molecular formula and chemical structure of complexes, etc., and achieve a wide range of applications. , the effect of broad action spectrum and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
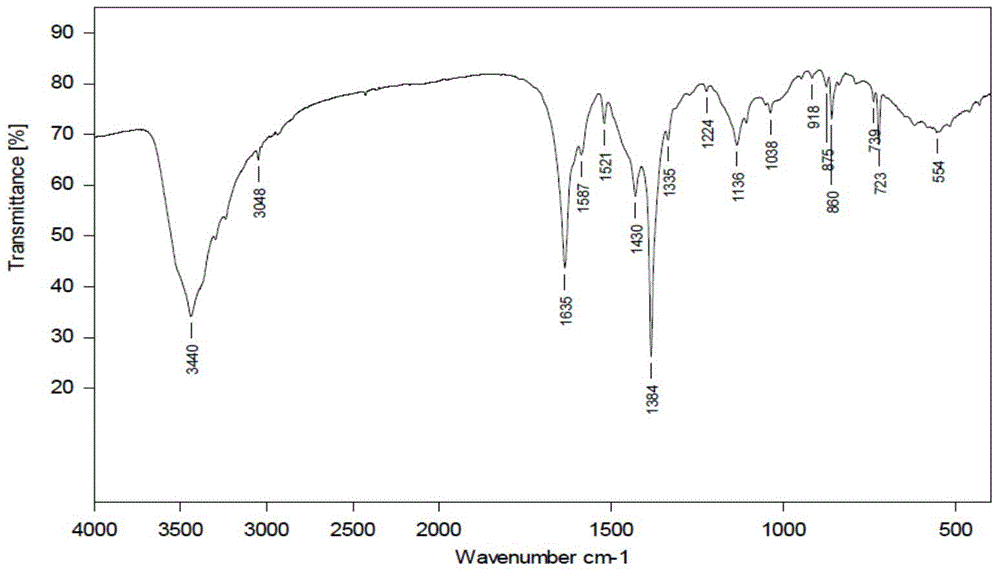
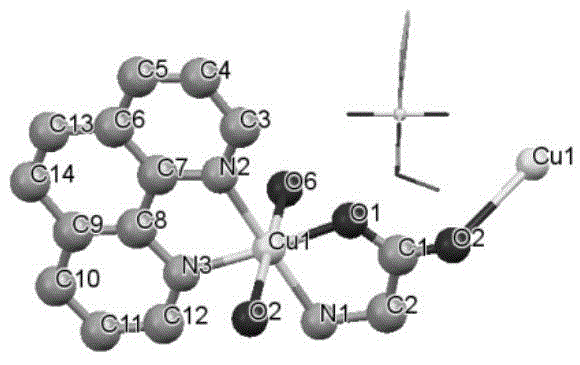
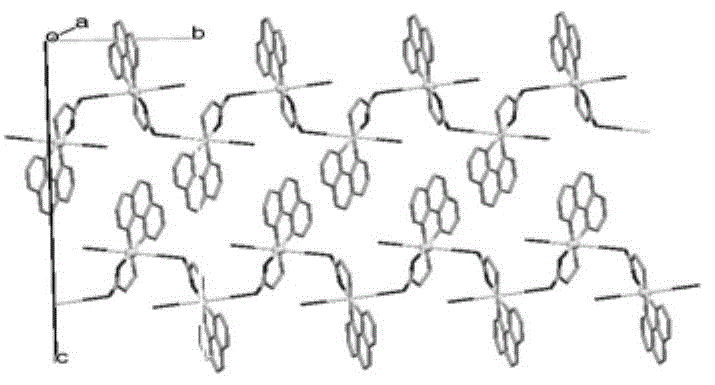
[0026] Example 1-1, preparation of complex Z1: Glycine (0.5mmol, 0.038g) and NaOH (0.5mmol, 0.02g) were mixed and dissolved in 30ml of ethanol and water mixed solvent (1:1), then Cu(NO 3 ) 2 ·3H 2 O (0.5mmol, 0.119g), stirred until fully dissolved; then added 1,10-phenanthroline (0.5mmol, 0.100g), stirred for 8 hours. Then filter and put the filtrate into a 20ml small test tube, add diethyl ether to the top of the filtrate (volume ratio 1-3), let it stand, and obtain blue crystals in about 20 days. Elemental analysis (%): calculated value (experimental value): C39.53 (39.60); H4.00 (4.01); N13.18 (13.15). Its infrared spectrum and X-diffraction single crystal structure diagram are shown in the attached Figure 1A , 1B and 1C. Z1 compound molecular formula is: {[Cu II (phen)(Gly)(H 2 O)] NO 3 - 2H 2 O} n , that is, 1 1,10-Phen, 1 glycine, 1 H 2 O coordinates with 1 Cu to form a repeating unit ( Figure 1B ). This repeating unit is bridged by Cu to form a one-dimen...
Embodiment 1-2: experiment 1-1,KOH(0.5mmol)NaOH(0.5mmol)。 Embodiment 1-3
[0028] Example 1-2: The experiment was the same as 1-1, except that KOH (0.5 mmol) replaced NaOH (0.5 mmol). Embodiment 1-3: experiment is the same as 1-1, just Cu(ClO 4 ) 2 ·6H 2 O (0.5mmol) and NaNO 3 (0.5mol) to replace Cu(NO 3 ) 2 ·3H 2 O (0.5 mmol). Embodiment 1-4: experiment is the same as 1-1, just Cu(SO 4 ) 2 ·5H 2 O (0.5mmol) and NaNO 3 (0.5mol) to replace Cu(NO 3 ) 2 3H 2 O (0.5 mmol). Embodiment 1-5: experiment is the same as 1-1, just CuCl 2 2H 2 O (0.5mmol) and NaNO 3 (0.5mol) to replace Cu(NO 3 ) 2 ·3H 2 O (0.5 mmol). Embodiment 1-6: experiment is the same as 1-1, just CuBr 2 (0.5mmol) and NaNO 3 (0.5mol) to replace Cu(NO 3 ) 2 ·3H 2 O (0.5 mmol). Embodiment 1-7: experiment is the same as 1-1, just Cu(CH 3 COOH) 2 ·H 2 O (0.5mmol) and NaNO 3 (0.5mol) to replace Cu(NO 3 ) 2 ·3H 2 O (0.5 mmol).
Embodiment 2-1
[0029] Example 2-1, preparation of complex Z2, L-phenylalanine (0.5mmol, 0.083g) and NaOH (0.5mmol, 0.02g) were mixed and dissolved in 30ml of ethanol and water mixed solvent (1:1) ; Then add CuCl 2 2H 2 O (0.5mmol, 0.0874g), then stirred until fully dissolved; then added 1,10-phenanthroline (0.5mmol, 0.099g), stirred for 12 hours; filtered, put the filtrate into a 20ml small test tube, and added diethyl ether To the top of the filtrate (volume ratio 1-3), let it stand, and obtain blue crystals after about a week. Elemental analysis (%): calculated value (experimental value): C50.71 (50.74); H4.86 (4.77); N8.45 (8.54). Its infrared spectrum and X-diffraction single crystal structure diagram are shown in the attached Figure 2A with 2B . Z2 compound molecular formula is: [Cu II (phen)(L-Phe)(Cl)] 2 ·6H 2 O, that is, 1 1,10-Phen, 1 phenylalanine, and 1 Cl coordinate with 1 Cu to form a cis complex; the other 1,10-Phen, 1 phenylalanine Acid, and 1 Cl coordinates with 1 C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com