Preparation method of acotiamide intermediate
A technology of acotiamide and intermediates, applied in directions such as organic chemistry, can solve problems such as inability to realize industrialized production, unfavorable large-scale production, expensive trifluoromethanesulfonate, etc., and achieve high implementation value and social, economic and environmental protection benefits, The effect of less waste, easy separation and reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
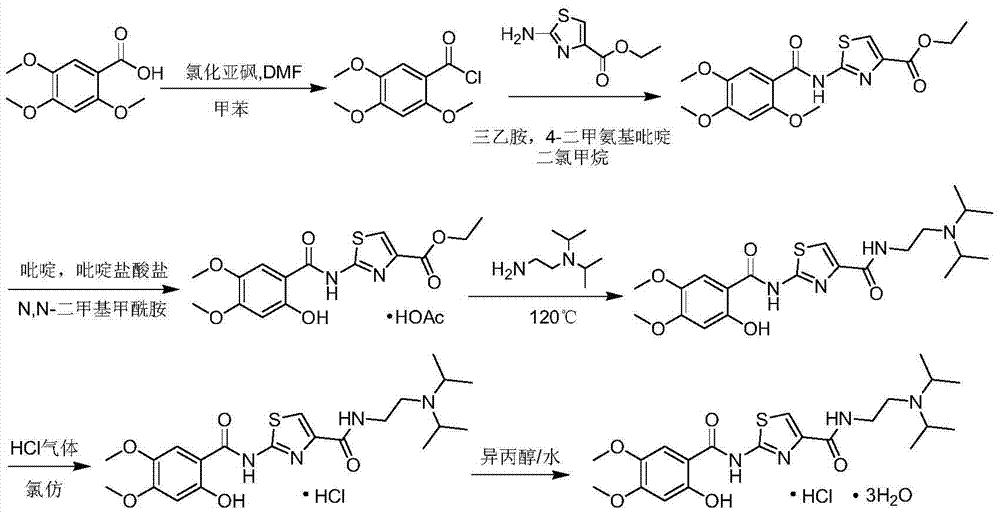
[0054] Preparation of Compound III: Add 2.12g (0.01mol) of II into the reaction flask, then add 1.66g (0.014mol) of thionyl chloride and 10.6ml of toluene, heat the system to reflux, keep the reaction for 1.5h, after the reaction is completed Concentrate under reduced pressure to obtain compound III;
[0055] Preparation of compound IV: Dissolve compound III (theoretical content of 0.01mol) obtained by concentration in tetrahydrofuran, and then add it dropwise to a reaction flask containing 0.84g (0.011mol) of thiourea in 16.0ml of tetrahydrofuran solution. , the temperature of the system was raised to reflux, and after the reaction was completed, the system was concentrated to obtain compound IV;
[0056] Preparation of Compound I: Dissolve Compound IV (theoretical content of 0.01mol) obtained by concentration in 25ml of methanol, then add ethyl bromopyruvate 2.05 (0.0105mol) and potassium carbonate 1.45g (0.0105), heat to reflux, keep Reflux reaction for 2.5±0.5h, evaporate...
Embodiment 2
[0059]Preparation of Compound III: Add 2.12g (0.01mol) of II into the reaction flask, then add 1.90g (0.016mol) of thionyl chloride and 11.6ml of toluene, heat the system to 80±5°C, react for 1.5h, and react Concentrate under reduced pressure after completion to obtain compound III;
[0060] Preparation of Compound IV: Dissolve Compound III (theoretical content of 0.01 mol) obtained by concentration in tetrahydrofuran, and then add it dropwise to a reaction flask containing 0.76 g (0.010 mol) of thiourea in 15.0 ml of tetrahydrofuran solution, and the addition is complete Afterwards, the temperature of the system was raised to reflux, TLC showed that the reaction was completed in 2 hours, and the system was concentrated to obtain compound IV;
[0061] Preparation of compound I: Dissolve compound IV (theoretical content of 0.01mol) obtained by concentration in 30ml of absolute ethanol, then add ethyl bromopyruvate 2.24 (0.0115mol) and potassium carbonate 1.59g (0.0115), and hea...
Embodiment 3
[0064] "One-pot method" to prepare compound Ⅰ: add 2.12g (0.01mol) of Ⅱ into the reaction flask, then add 1.78g (0.015mol) of thionyl chloride and 10.6ml of toluene, heat the system to 80±5℃, and keep it warm React for 1.5h, after the reaction is completed, concentrate under reduced pressure to obtain compound III; dissolve the concentrated compound III in tetrahydrofuran, and then add it dropwise to a reaction flask containing 0.80 g (0.0105 mol) of thiourea in 16.0 ml of tetrahydrofuran solution, drop After the addition, the system was heated to reflux. After the reaction was completed, the system was concentrated to obtain compound IV; the concentrated compound IV was dissolved in 25ml of absolute ethanol, and then ethyl bromopyruvate 2.15 (0.011mol) and carbonic acid were added Potassium 1.52g (0.011mol), heat to reflux, keep reflux for 1h, distill anhydrous alcohol under reduced pressure, add water to stir, filter, wash with water to obtain the crude product; disperse the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com