2-ethyl-1,3-hexanediol purifying process and related substance detecting method
A detection method and purification method technology, applied in the field of medicine, can solve the problems of product quality impact, impact on the application of injection-grade excipients, etc., and achieve the effects of strict and accurate control, reducing the generation of impurities and ensuring quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
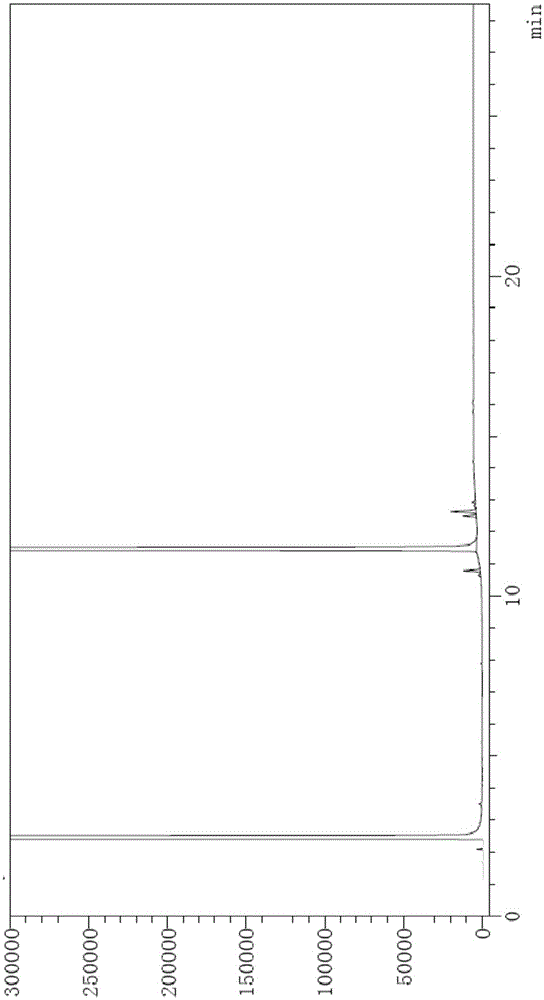
[0099] Chromatographic conditions: DB624 chromatographic column with (6%) cyanopropylphenyl-(94%) methylpolysiloxane as the stationary liquid, the specification is 30m×0.53mm×3μm; the initial column temperature is 70°C, and it is maintained for 3 Minutes, the temperature was raised to 260°C at a rate of 20°C per minute, and maintained for 16 minutes; the split ratio was 10:1, the flow rate was 3ml / min, the inlet temperature was 250°C, and the detector temperature was 275°C.
[0100] Step 1, preparation of system suitability solution: the method is as follows: Weigh 2-ethyl-1,3-hexanediol and an appropriate amount of impurity 1, impurity 2, and impurity 3, and add absolute ethanol to prepare 2-ethyl-1 , The solution of 20mg / ml of 3-hexanediol and 0.1mg / ml of impurity 1, impurity 2, and impurity 3 is used as the system suitability solution;
[0101] Step 2, preparation of the test solution: the method is as follows: Weigh an appropriate amount of 2-ethyl-1,3-hexanediol, add abso...
Embodiment 2
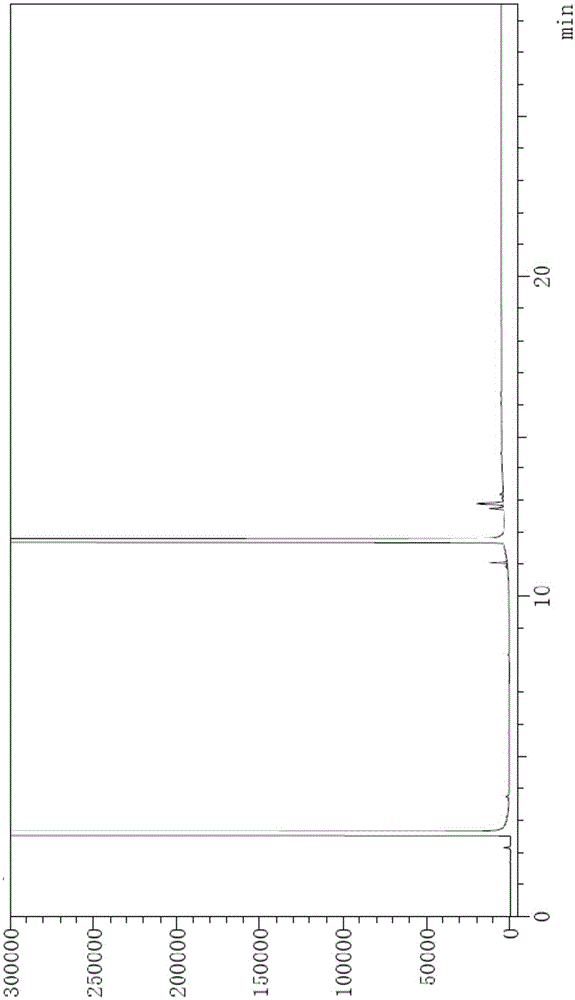
[0107] Chromatographic conditions: DB624 chromatographic column with (6%) cyanopropylphenyl-(94%) methylpolysiloxane as the stationary liquid, the specification is 30m × 0.53mm × 3μm; the initial column temperature is 65 ° C, maintain 3 Minutes, the temperature was raised to 260°C at a rate of 20°C per minute, and maintained for 16 minutes; the split ratio was 10:1, the flow rate was 3ml / min, the inlet temperature was 250°C, and the detector temperature was 275°C.
[0108] Step 1, preparation of system suitability solution: the method is as follows: Weigh 2-ethyl-1,3-hexanediol and an appropriate amount of impurity 1, impurity 2, and impurity 3, and add absolute ethanol to prepare 2-ethyl-1 , The solution of 20mg / ml of 3-hexanediol and 0.1mg / ml of impurity 1, impurity 2, and impurity 3 is used as the system suitability solution;
[0109] Step 2, preparation of the test solution: the method is as follows: Weigh an appropriate amount of 2-ethyl-1,3-hexanediol, add absolute ethan...
Embodiment 3
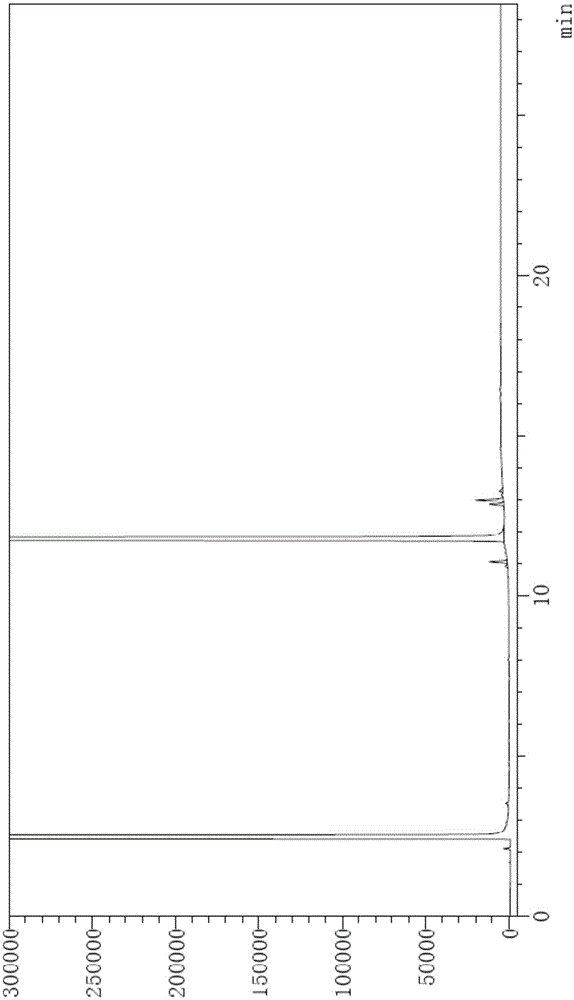
[0115]Chromatographic conditions: DB624 chromatographic column with (6%) cyanopropylphenyl-(94%) methylpolysiloxane as the stationary liquid, the specification is 30m×0.53mm×3μm; the initial column temperature is 70°C, and it is maintained for 3 minutes, the temperature was raised to 260°C at a rate of 19°C per minute, and maintained for 16 minutes; the split ratio was 10:1, the flow rate was 3ml / min, the inlet temperature was 250°C, and the detector temperature was 275°C.
[0116] Step 1, preparation of system suitability solution: the method is as follows: Weigh 2-ethyl-1,3-hexanediol and an appropriate amount of impurity 1, impurity 2, and impurity 3, and add absolute ethanol to prepare 2-ethyl-1 , The solution of 20mg / ml of 3-hexanediol and 0.1mg / ml of impurity 1, impurity 2, and impurity 3 is used as the system suitability solution;
[0117] Step 2, preparation of the test solution: the method is as follows: Weigh an appropriate amount of 2-ethyl-1,3-hexanediol, add absol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com